Structure Of The Carbonyl Group
- The carbonyl carbon atom is sp2-hybridised and forms three sigma bonds. The fourth valence electron of carbon remains in its p-orbital and forms a π-bond with oxygen by overlapping with the p-orbital of an oxygen.
- The oxygen atom also has two non-bonding electron pairs. Thus, the carbonyl carbon and the three atoms attached to it lie in the same plane, and the p-electron cloud is above and below this plane.
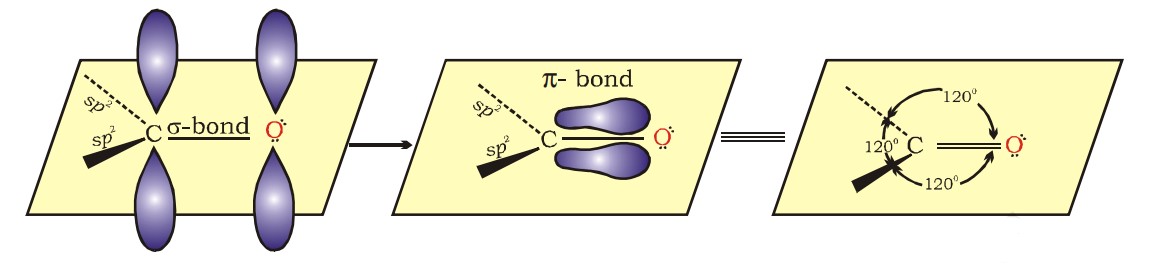
- The bond angles are approximately 120°, as expected of a trigonal coplanar structure


Preparation Of Aldehydes And Ketones (I)
(i) Oxidation of alcohols involves the formation of a carbon-oxygen double bond, leading to a Dehydrogenation reaction.
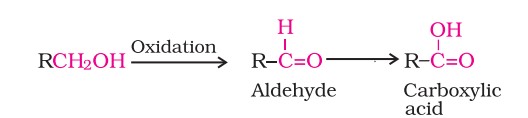
(ii) Strong oxidising agents such as acidified potassium permanganate are used for getting carboxylic acids from alcohols directly. CrO3 in anhydrous medium is used as the oxidising agent for the isolation of aldehydes.
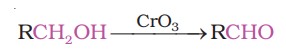
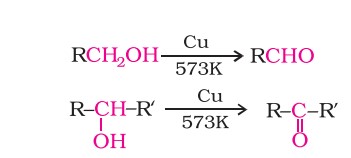
(iii) When the vapours of a primary or a secondary alcohol are passed over heated copper at 573 K, dehydrogenation takes place and an aldehyde or a ketone is formed.


Preparation Of Aldehydes And Ketones (II)
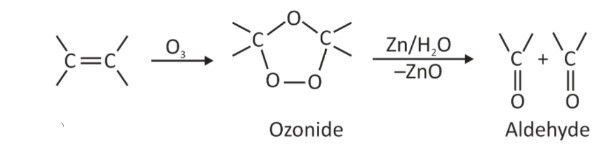
(i) By ozonolysis of alkenes: As we know, ozonolysis of alkenes, followed by reaction with zinc dust and water, gives aldehydes, ketones or a mixture of both, depending on the substitution pattern of the alkene.
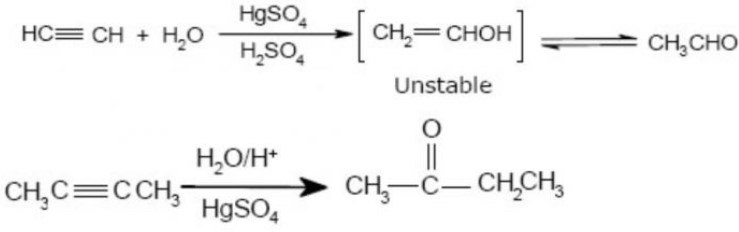
(ii) By hydration of alkynes: The addition of water to ethyne in the presence of H2SO4 and HgSO4 gives acetaldehyde. All other alkynes give ketones in this reaction.


Preparation Of Aldehydes (I)
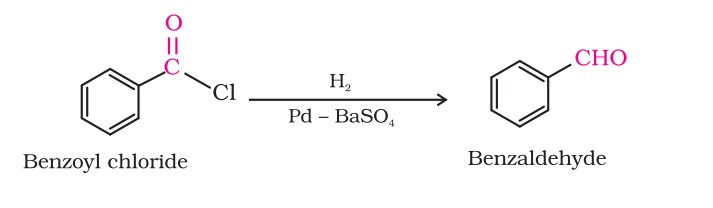
1. From acyl chloride (acid chloride)
Acyl chloride (acid chloride) is hydrogenated over catalyst, palladium on barium sulphate. This reaction is called Rosenmund reduction.


Preparation Of Aldehydes (II)
Stephen reaction - Nitriles are reduced to the corresponding imine with stannous chloride in the presence of hydrochloric acid, which on hydrolysis gives the corresponding aldehyde.

Alternatively, nitriles are selectively reduced by diisobutylaluminium hydride (DIBAL-H) to imines, followed by hydrolysis to aldehydes:
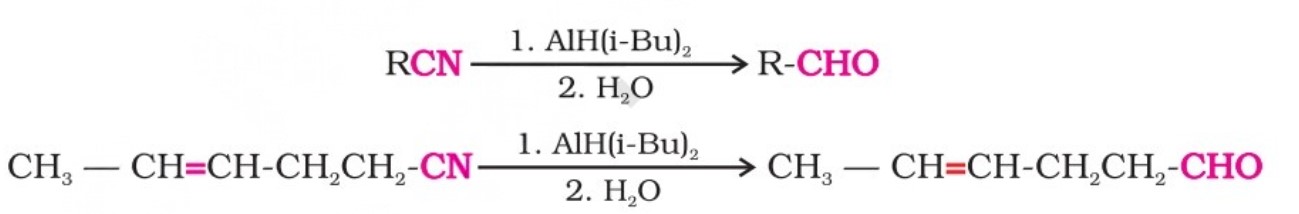
Similarly, esters are also reduced to aldehydes with DIBAL-H.
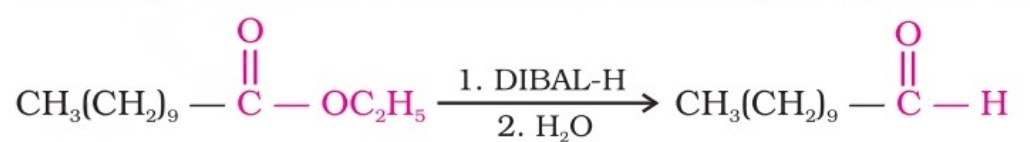


Preparation Of Aldehydes (III)
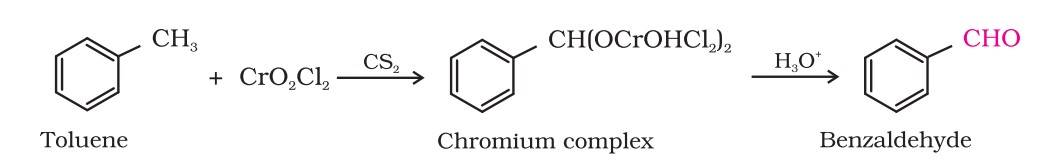
(i) Etard reaction : Chromyl chloride oxidises the methyl group to a chromium complex, which on hydrolysis gives the corresponding benzaldehyde.
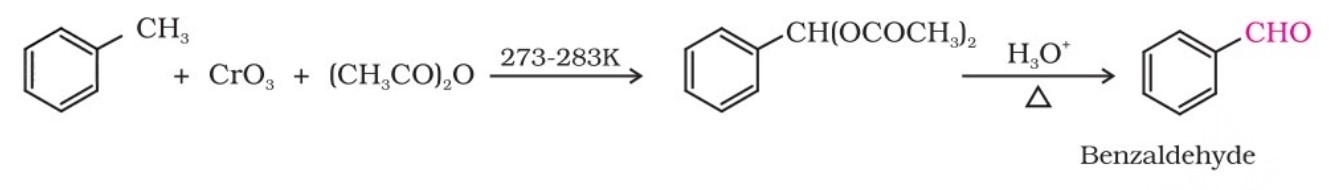
(ii) Use of chromic oxide (CrO3) : Toluene or substituted toluene is converted to benzylidene diacetate on treating with chromic oxide in acetic anhydride. The benzylidene diacetate can be hydrolysed to the corresponding benzaldehyde with aqueous acid.


Preparation Of Aldehydes (IV)
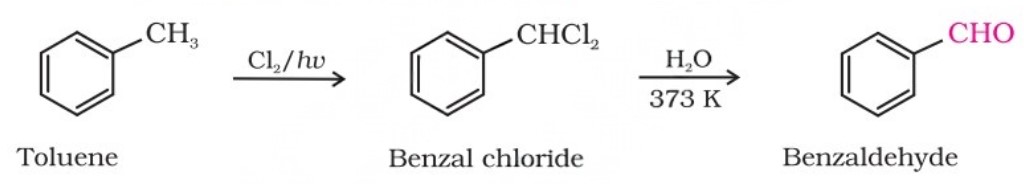
(iii) By side chain chlorination followed by hydrolysis : This method gives benzal chloride, which on hydrolysis gives benzaldehyde.
(iv) By Gatterman–Koch reaction : When benzene or its derivatives are treated with carbon monoxide and hydrogen chloride in the presence of anhydrous aluminium chloride or cuprous chloride, it gives benzaldehyde or substituted benzaldehyde.
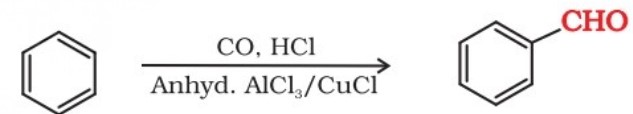


Preparation Of Ketones (I)
(i) From acyl chlorides : Treatment of acyl chlorides with dialkylcadmium, prepared by the reaction of cadmium chloride with Grignard reagent, gives ketones.
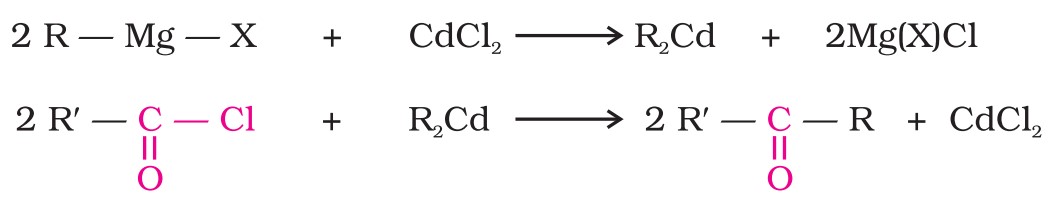
2. From nitriles : Treating a nitrile with a Grignard reagent followed by hydrolysis yields a ketone.
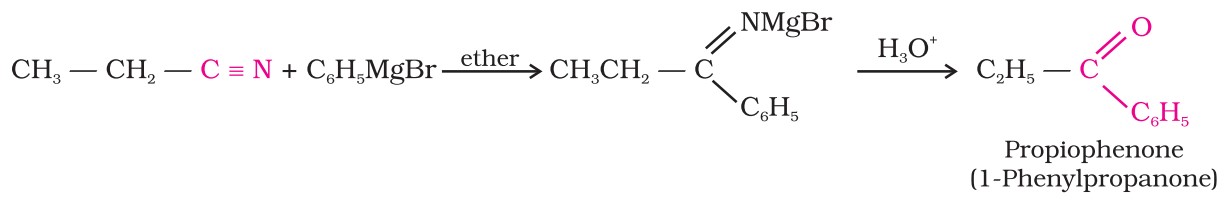


Preparation Of Ketones (II)
(iii) From benzene or substituted benzenes : When benzene or substituted benzene is treated with acid chloride in the presence of anhydrous aluminium chloride, it affords the corresponding ketone.

This reaction is known as the Friedel-Crafts acylation reaction.


Physical Properties Of Aldehydes And Ketones
(i) Boiling points of aldehydes and ketones are higher than those of non-polar compounds, hydrocarbons and ethers of comparable molecular masses due to dipole-dipole interactions between the opposite ends of the C=O dipoles.
Example of order of boiling point -
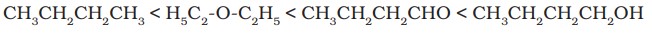
(ii) Solubility decreases with an increase in molecular mass. Lower aldehydes and ketones containing up to four carbon atoms are soluble in water.


Methods Of Preparation Of Carboxylic Acids (I)
(i) From primary alcohols and aldehydes: Primary alcohols are readily oxidised to carboxylic acids with common oxidising agents such as potassium permanganate (KMnO4 ) in neutral, acidic or alkaline media or by potassium dichromate and chromium trioxide in acidic media (Jones reagent).
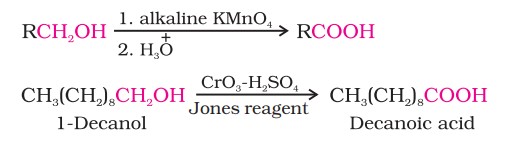


Methods Of Preparation Of Carboxylic Acids (II)
(ii) From alkylbenzenes: Aromatic carboxylic acids can be prepared by vigorous oxidation of alkylbenzenes with chromic acid or acidic or alkaline potassium permanganate.
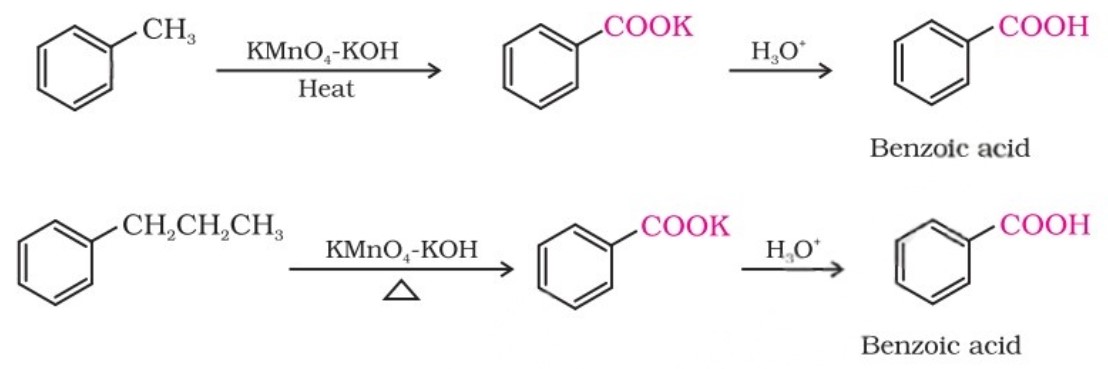 Primary and secondary alkyl groups are oxidised in this manner, while the tertiary group is not affected.
Primary and secondary alkyl groups are oxidised in this manner, while the tertiary group is not affected.


Methods Of Preparation Of Carboxylic Acids (III)
(iii) From nitriles and amides: Nitriles are hydrolysed to amides and then to acids in the presence of acid or base as a catalyst. Mild reaction conditions are used to stop the reaction at the amide stage.



Methods Of Preparation Of Carboxylic Acids (IV)
(iv) From Grignard reagents : Grignard reagents react with carbon dioxide (dry ice) to form salts of carboxylic acids, which in turn give corresponding carboxylic acids after acidification with mineral acid.
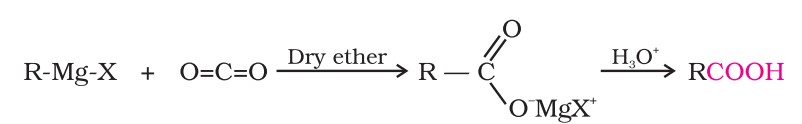
(v) From acyl halides and anhydrides : Acid chlorides, when hydrolysed with water, give carboxylic acids or, more readily, hydrolysed with aqueous base to give carboxylate ions, which on acidification, provide corresponding carboxylic acids.
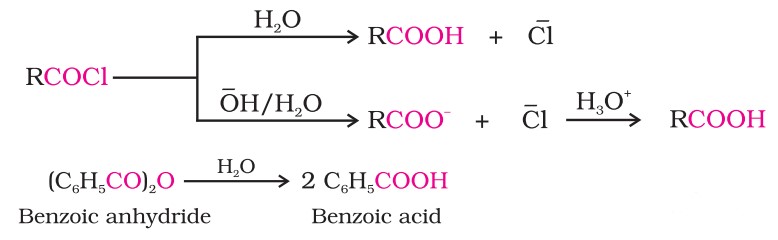


Methods Of Preparation Of Carboxylic Acids (V)
From esters : Acidic hydrolysis of esters gives directly carboxylic acids, while basic hydrolysis gives carboxylates, which on acidification give corresponding carboxylic acids.
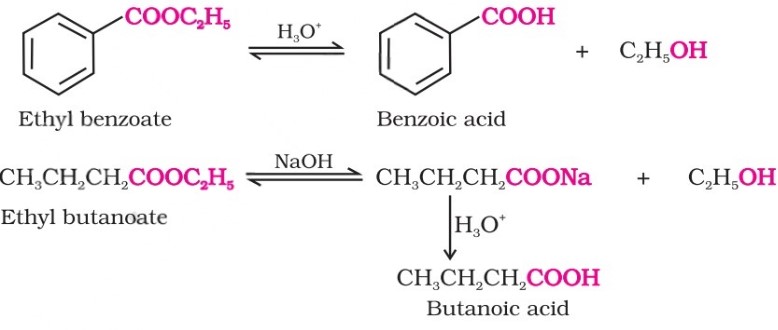


Physical Properties Of Carboxylic Acids
(i) The first three members of this series are colourless, pungent-smelling liquids.
(ii) Carboxylic acids exist in the form of a dimer due to hydrogen bonding.
(iii) The solubility of carboxylic acids in water decreases due to an increase in the size of the hydrophobic hydrocarbon part. Lower carboxylic acids having up to four carbon atoms are miscible in water due to the formation of hydrogen bonds with water.
(iv) The Boiling point of carboxylic acids is higher than those of hydrocarbons, aldehydes, ketones and alcohols of comparable molecular masses due to intermolecular H-bonding as shown in the figure.
(v) Melting points of carboxylic acids increase with an increase in their molecular mass.


Chemical Reactions - Introduction
Since aldehydes and ketones both possess the carbonyl functional group, they undergo similar chemical reactions.
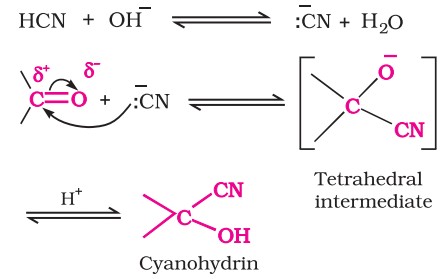
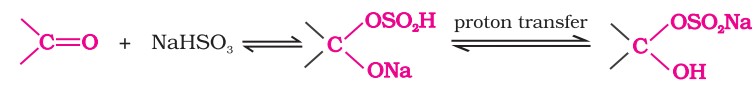
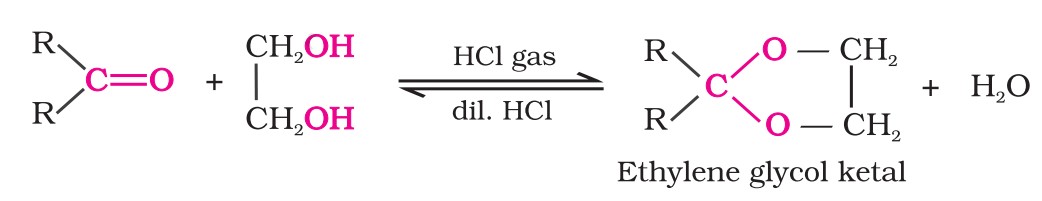
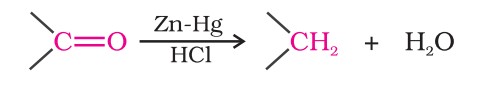
1. Nucleophilic addition reactions
(i) Mechanism of nucleophilic addition reactions - A nucleophile attacks the electrophilic carbon atom of the polar carbonyl group from a direction approximately perpendicular to the plane of sp2 hybridised orbitals of carbonyl carbon. The hybridisation of carbon changes from sp2 to sp3 in this process.
(ii) Reactivity - Aldehydes are generally more reactive than ketones in nucleophilic addition reactions due to steric and electronic reasons. Sterically, the presence of two relatively large substituents in ketones hinders the approach of nucleophile to carbonyl carbon than in aldehydes having only one such substituent.


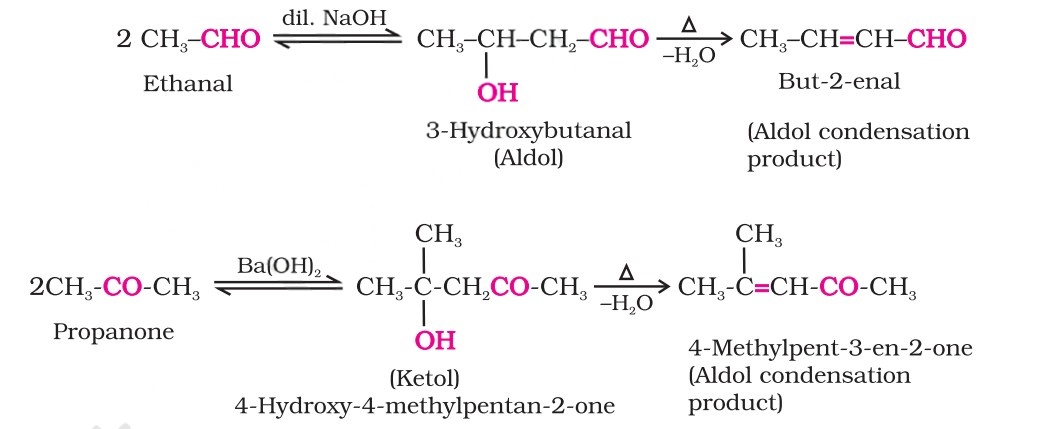
Aldol Condensation
Aldehydes and ketones having at least one α-hydrogen undergo a reaction in the presence of dilute alkali as a catalyst to form β-hydroxy aldehydes (aldol) or β-hydroxy ketones (ketol), respectively. This is known as the Aldol reaction.


Oxidation Reactions (II)
(iii) Oxidation of methyl ketones by haloform reaction: Aldehydes and ketones having at least one methyl group linked to the carbonyl carbon atom (methyl ketones) are oxidised by sodium hypohalite to sodium salts of corresponding carboxylic acids having one carbon atom less than that of the carbonyl compound.
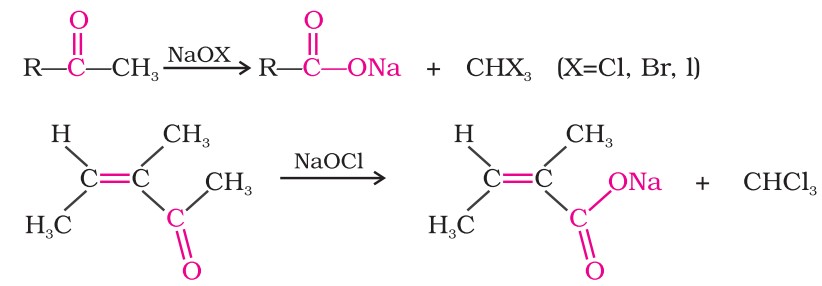
The methyl group is converted to haloform.


Oxidation Reactions (I)
(i) Tollens’ test: On warming an aldehyde with freshly prepared ammoniacal silver nitrate solution (Tollens’ reagent), a bright silver mirror is produced due to the formation of silver metal. 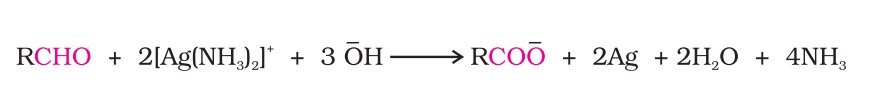
(ii) Fehling’s test: On heating an aldehyde with Fehling’s reagent, a reddish brown precipitate is obtained. Aldehydes are oxidised to the corresponding carboxylate anion. Aromatic aldehydes do not respond to this test.



Cannizzaro Reaction
Aldehydes, which do not have an α-hydrogen atom, undergo self-oxidation and reduction (disproportionation) reaction on heating with concentrated alkali.
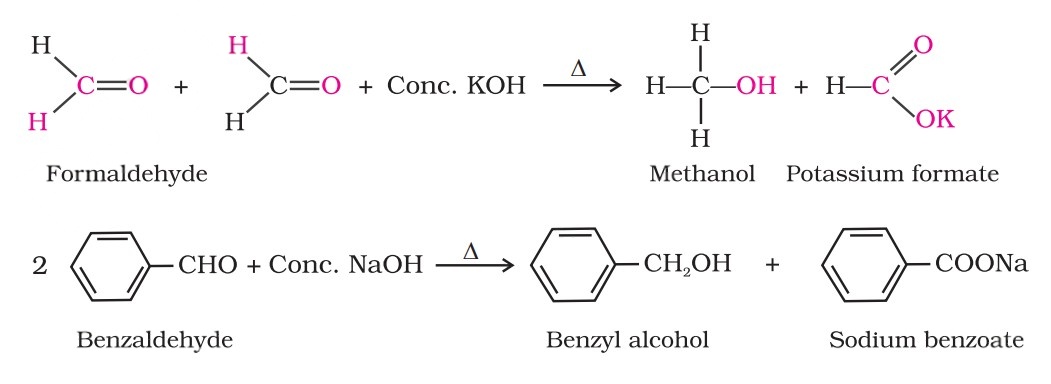 In this reaction, one molecule of the aldehyde is reduced to alcohol while another is oxidised to carboxylic acid salt.
In this reaction, one molecule of the aldehyde is reduced to alcohol while another is oxidised to carboxylic acid salt.


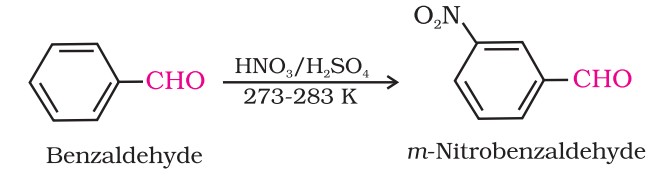
Electrophilic Substitution Reaction - Aldehydes And Ketones
Aromatic aldehydes and ketones undergo electrophilic substitution at the ring in which the carbonyl group acts as a deactivating and meta-directing group.


Chemical Reactions - Carboxylic Acids (I)
(i) Acidity
The carboxylic acids like alcohols evolve hydrogen with electropositive metals and form salts with alkalies similar to phenols.



Chemical Reactions - Carboxylic Acids (II)
(ii) Formation of anhydride
Carboxylic acids, when heated with mineral acids such as Sulphuric Acid, yield the corresponding anhydride.
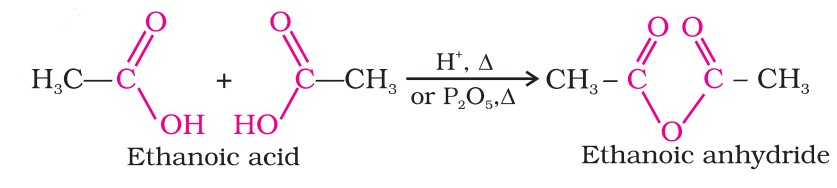
(iii) Esterification
Carboxylic acids are esterified with alcohols or phenols in the presence of a mineral acid such as concentrated Sulphuric acid or HCl gas as a catalyst.
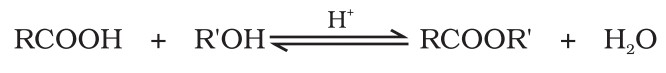


Chemical Reactions - Carboxylic Acids (III)
(iv) Reaction with ammonia
Carboxylic acids react with ammonia to give ammonium salt which on further heating at high temperature, gives amides.



Chemical Reactions - Carboxylic Acids (IV)
(v) Hell-Volhard-Zelinsky reaction (Halogenation)
Carboxylic acids having an α-hydrogen are halogenated at the α-position on treatment with chlorine or bromine in the presence of a small amount of red phosphorus to give α-halocarboxylic acids.



Chemical Reactions - Carboxylic Acids (V)
(vi) Ring substitution
Aromatic carboxylic acids undergo electrophilic substitution reactions in which the carboxyl group acts as a deactivating and meta-directing group.
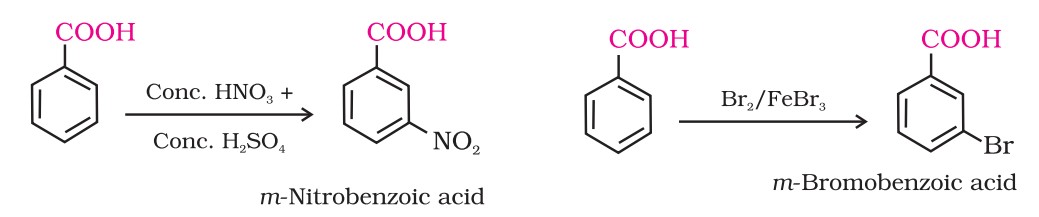
They, however, do not undergo Friedel-Crafts reaction (because the carboxyl group is deactivating and the catalyst, aluminium chloride (Lewis acid) gets bonded to the carboxyl group).


Chemical Reactions - Carboxylic Acids (VI)
(vii) Reduction
Carboxylic acids are reduced to primary alcohols by lithium aluminium hydride or, better, with diborane. Diborane does not easily reduce functional groups such as ester, nitro, halo, etc. Sodium borohydride does not reduce the carboxyl group.
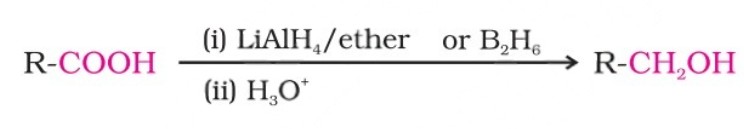
(viii) Decarboxylation - Kolbe Electrolysis
Carboxylic acids lose carbon dioxide to form hydrocarbons when their sodium salts are heated with sodalime (NaOH and CaO in the ratio of 3 : 1).
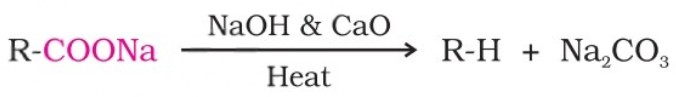


Chemical Reactions - Carboxylic Acids (VII)
(viii) Reactions with PCl5, PCl3 and SOCl2
The hydroxyl group of carboxylic acids behaves like that of alcohols and is easily replaced by a chlorine atom on treating with these substances.
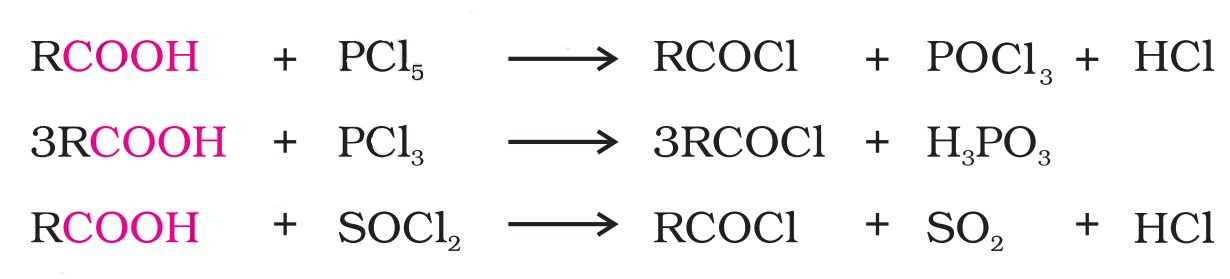 Thionyl chloride is preferred because the other two products are gaseous and escape the reaction mixture, making the purification of the products easier.
Thionyl chloride is preferred because the other two products are gaseous and escape the reaction mixture, making the purification of the products easier.


 beeTokens
beeTokens