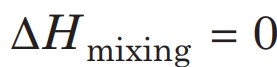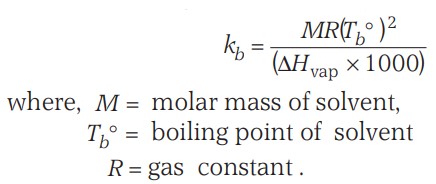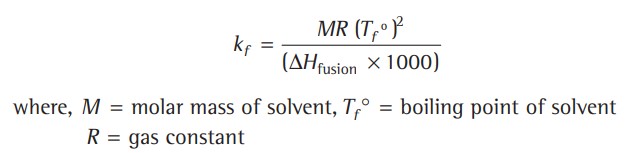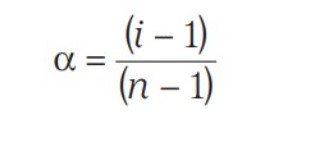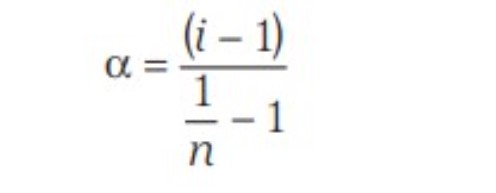Solutions And It's Types
Solutions are homogeneous mixtures of two or more components.
Generally, the component in the largest quantity is known as the solvent. Solvent determines the physical state in which the solution exists. One or more components present in the solution other than the solvent are called solutes.
Binary solutions - Solutions consisting of two components.
| Type of solution | Solute | Solvent | Examples |
| Gaseous Solutions | Gas | Gas | Mixture of oxygen and nitrogen gases |
| Liquid | Gas | Chloroform mixed with nitrogen gas | |
| Solid | Gas | Camphor in nitrogen gas | |
| Liquid Solutions | Gas | Liquid | Oxygen dissolved in water |
| Liquid | Liquid | Ethanol dissolved in water | |
| Solid | Liquid | Glucose dissolved in water | |
| Solid Solutions | Gas | Solid | Solution of hydrogen in palladium |
| Liquid | Solid | Amalgam of mercury with sodium | |
| Solid | Solid | Copper dissolved in gold |


Concentration Terms
A solution can be defined in several ways quantitatively.
These terms include -
- Mass percentage (w/w%)
- Volume percentage (V/V%)
- Mass by volume percentage (w/V%)
- Parts per million (ppm)
- Mole fraction (x)
- Molarity (M)
- Molality (m)


Mass Percentage And Volume Percentage
The mass percentage of a component of a solution is defined as:

Example - 10% w/w glucose solution in water results in a 100g solution where 10g of glucose is dissolved in 90g of water.
The volume percentage is defined as:

Example: A 10% ethanol solution in water means that 10ml of ethanol is dissolved in 90ml of water, resulting in a total volume of 100ml.


Mass By Volume Percentage And Parts Per Million
The mass by volume percentage of a component of a solution is defined as:

It can also be defined in simple terms as the mass of solute dissolved in 100ml of the solution.
This is commonly used in medicine and pharmacy.
The parts per million (ppm) of a component of a solution is useful when the solute is present in trace quantities, and is defined as:

A litre of sea water (which weighs 1030 g) contains about 0.006 g of dissolved oxygen. Such a small concentration is also expressed as 5.8 ppm of seawater.
The concentration of pollutants in water or atmosphere is often expressed in terms of µg/ml or ppm.


Mole Fraction
The mole fraction of a component of a solution is defined as:
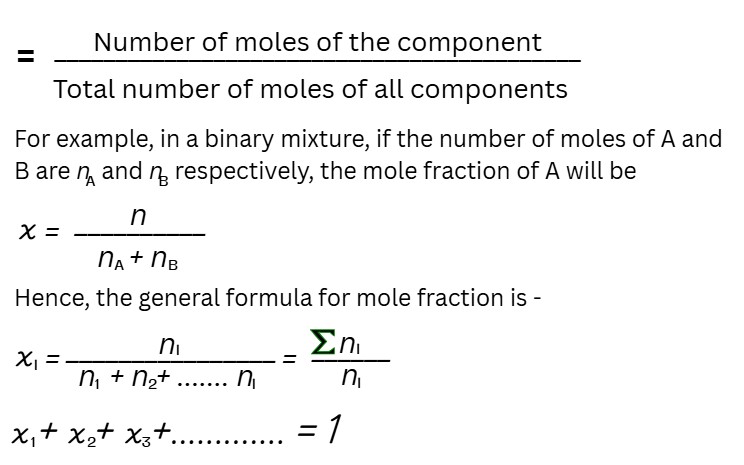
The mole fraction unit is very useful in relating some physical properties of solutions, like vapour pressure, to the concentration of the solution and calculations involving gas mixtures.


Molarity And Molality
The molarity of a component of a solution is defined as:

Example - 0.25 M solution of NaOH means that 0.25 moles of NaOH has been dissolved in one litre of water.
The molality of a component of a solution is defined as:
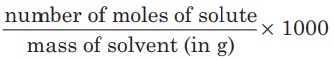
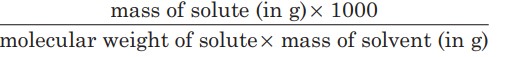
Molality is independent of temperature.
Unit of molality = g moles/kg of solvent.
Example - 1.00 mol/kg (or 1.00 m) solution of KCl means that 1 mol (74.5 g) of KCl is dissolved in 1 kg of water.


Solubility
Solubility of a substance is its maximum amount that can be dissolved in a specified amount of solvent at a specified temperature.
It depends upon the -
- Nature of Solute
- Nature of Solvent
- Temperature
- Pressure
Such a solution in which no more solute can be dissolved at the same temperature and pressure is called a saturated solution.
An unsaturated solution is one in which more solute can be dissolved at the same temperature.


Solubility Of A Solid In A Liquid
It is observed that polar solutes dissolve in polar solvents and non-polar solutes in non-polar solvents, thus, we may say like dissolves like.
- When a solid solute is added to the solvent, some solute dissolves and its concentration increases in the solution. This process is known as dissolution.
- Some solute particles in solution collide with the solid solute particles and get separated from the solution. This process is known as crystallisation.
A stage is reached when the two processes occur at the same rate. Thus, a state of dynamic equilibrium is reached.
Solute + Solvent ⇌ Solution
At this stage the concentration of solute in solution will remain constant under the given conditions, i.e., temperature and pressure.


Solubility Of Solids In Liquids
The solution in dynamic equilibrium with undissolved solute is the saturated solution and it contains the maximum amount of solute dissolved in a given amount of solvent. Thus, the concentration of solute in such a solution is its solubility.
1. Effect of temperature
The solubility of a solid in a liquid is significantly affected by temperature changes and follows the Le-Chatelier's Principle.
- If in a nearly saturated solution, the dissolution process is endothermic (ΔH > 0), the solubility should increase with temperature rise
- If in a nearly saturated solution, the dissolution process is exothermic (ΔH < 0), the solubility should decrease with temperature rise.
2. Effect of pressure
Pressure does not have any significant effect on the solubility of solids in liquids because solids and liquids are highly incompressible and practically remain unaffected by changes in pressure.


Solubility Of A Gas In A Liquid And Henry's Law
The solubility of gases increases with an increase in pressure and a decrease in temperature.
Henry was the first to give a quantitative relation between pressure and solubility of a gas in a solvent, known as Henry’s law.
Henry's law states that - "At a constant temperature, the solubility or the mole fraction of a gas in the solution is directly proportional to the partial pressure of the gas present above the surface of the liquid or solution."
It is also expressed as-
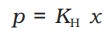
p = Partial pressure
x = mole fraction of the gas
Kh = Henry's constant


Henry's Law (Graph Analysis)
If we draw a graph between the partial pressure of the gas versus the mole fraction of the gas in solution -
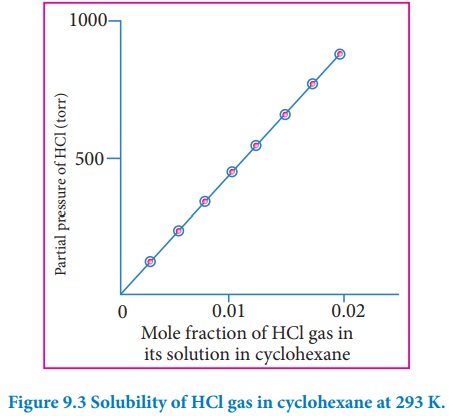
- Different gases have different KH values at the same temperature. Thus, KH is a function of the nature of the gas.
- Also, the higher the value of KH at a given pressure, the lower is the solubility of the gas in the liquid.
- As the temperature increases, the value of KH increases, indicating that the solubility of gases increases with a decrease in temperature.
It is due to this reason that aquatic species are more comfortable in cold waters than in warm waters.


Applications Of Henry's Law
Applications of Henry’s law -
- To increase the solubility of carbon dioxide in soft drinks and soda water, the bottle is sealed under high pressure.
- Scuba divers must cope with high concentrations of dissolved gases while breathing air at high pressure underwater which increases the solubility of atmospheric gases in blood, and when they come towards the surface, the pressure gradually decreases. This releases the dissolved gases and leads to the formation of bubbles of nitrogen in the blood, blocking capillaries and creating a medical condition known as bends. To avoid bends, the tanks used by scuba divers are filled with air diluted with helium (11.7% helium, 56.2% nitrogen and 32.1% oxygen).
- At high altitudes, the partial pressure of oxygen is less than that at ground level leading low concentrations of oxygen in the blood and tissues of people living at high altitudes or climbers. Low blood oxygen causes climbers to become weak and unable to think clearly, symptoms of a condition known as anoxia.
Effects of temperature
Solubility of gases in liquids decreases with a rise in temperature.
Following Le Chatelier's principle, as dissolution is an exothermic process, the solubility should decrease with the increase in temperature.


Raoult's Law
Raoult’s law, which states that for a solution of volatile liquids, the partial vapour pressure of each component of the solution is directly proportional to its mole fraction present in solution. Thus, for component 1 -
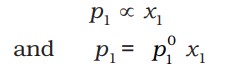
Similarly, for component 2 -
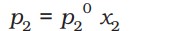
According to Dalton’s law of partial pressures, the total pressure over the solution phase in the container will be the sum of the partial pressures of the components of the solution -
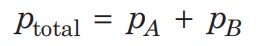
Substituting the values, we get -
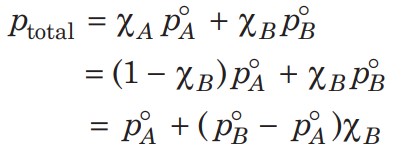
The following conclusions can be drawn from the equation-
- Total vapour pressure over the solution can be related to the mole fraction of any one component.
- Total vapour pressure over the solution varies linearly with the mole fraction of component 2.


Raoult's Law Graph And Dalton's Law
From the following plot, we can comprehend that -
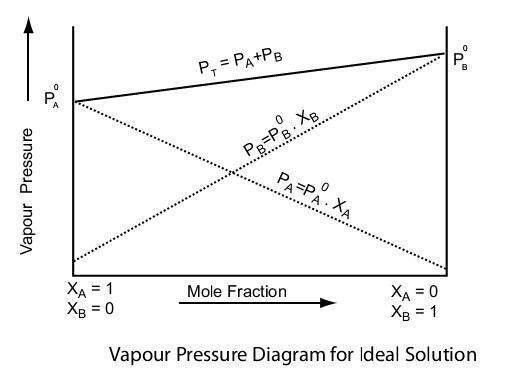
- The Minimum of P (total) is at Pº(A)
- The maximum of P (total) is at Pº(B)
- Solution B is more volatile than A because Pº(A) < Pº(B)
_________________________________________________________________
The partial pressures of the components determine the composition of the vapour phase in equilibrium with the solution.
If y1 and y2 are the mole fractions of the components 1 and 2, respectively, in the vapour phase, then, using Dalton’s law of partial pressures:
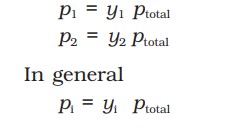


Vapour Pressure Of Solutions Of Solids In Liquids
In a pure liquid, the entire surface is occupied by the molecules of the liquid. If a non-volatile solute is added to a solvent to give a solution, the vapour pressure of the solution is solely from the solvent alone.
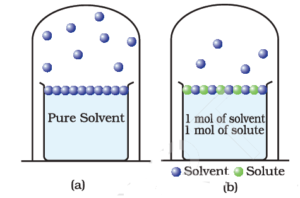
In the solution, the surface has both solute and solvent molecules; thereby, the fraction of the surface covered by the solvent molecules gets reduced. Hence, the number of solvent molecules escaping from the surface is correspondingly reduced, thus, the vapour pressure is also reduced.
The decrease in the vapour pressure of solvent depends on the quantity of non-volatile solute present in the solution, irrespective of its nature.
For example, the decrease in the vapour pressure of the same quantity of water (taken athe t same temperature) by adding 1.0 mol of sucrose ≈ 1.0 mol of urea


Ideal Solutions
Definition - The solutions that obey Raoult’s law over the entire range of concentration are known as ideal solutions.
- In an ideal solution of two components, say A and B, all cohesive forces (A-A, B-B, and A-B ) must be identical.
- Both have a similar structure
- Both have similar molecular sizes
- Both have identical intermolecular forces.
Its characteristics include -
Examples of the ideal solutions -
- Benzene-toluene
- n-hexane-n-heptane
- ethyliodide-ethyl bromide
- chlorobenzene- bromobenzene.


Non-ideal Solutions
Definition - When a solution does not obey Raoult’s law over the entire range of concentration, then it is called a non-ideal solution.
The vapour pressure of such a solution is either higher or lower than that predicted by Raoult’s law. If it is higher, the solution exhibits positive deviation, and if it is lower, it exhibits negative deviation from Raoult’s law.
The plots of vapour pressure as a function of mole fractions for such solutions are shown below.
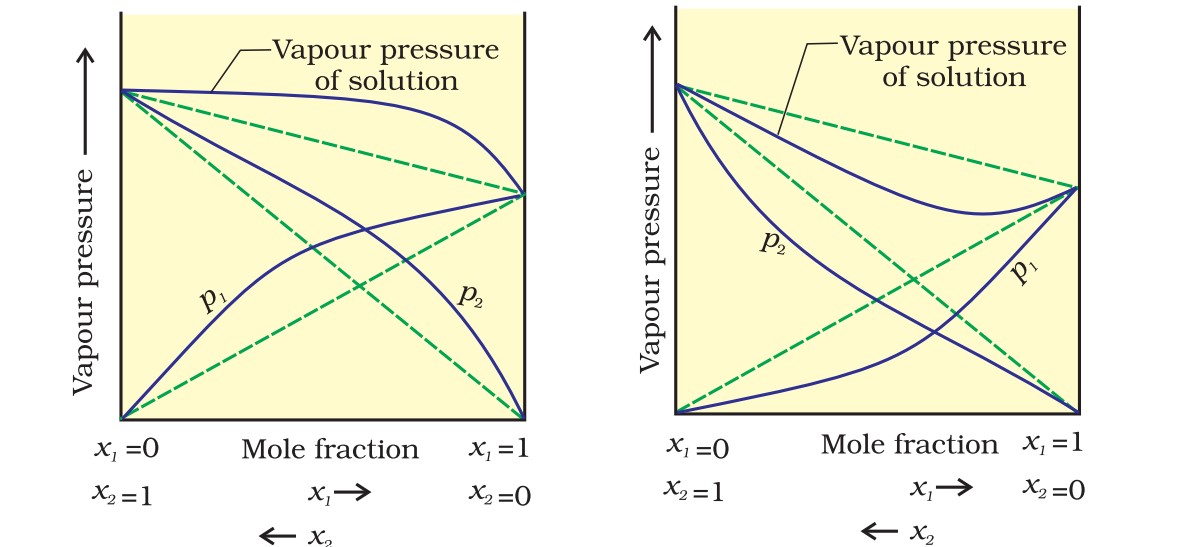
The cause for these deviations lie in the nature of interactions at the molecular level.


Positive Deviation
In case of positive deviation from Raoult’s law, A-B interactions are weaker than those between A-A or B-B, i.e., in this case, the intermolecular attractive forces between the solute-solvent molecules are weaker than those between the solute-solute and solvent-solvent molecules.
This means that in such solutions, molecules of A (or B) will find it easier to escape than in the pure state. This will increase the vapour pressure and result in positive deviation.
Example 1 - Mixtures of ethanol and acetone behave in this manner. In pure ethanol, molecules are hydrogen-bonded. On adding acetone, its molecules get in between the host molecules and break some of the hydrogen bonds between them. Due to weakening of interactions, the solution shows positive deviation.
Other examples -
- Acetone and benzene
- Carbon disulfide and acetone
- Water and methanol
- Water and ethanol
- Benzene and methanol
- Ethanol and chloroform
- Acetic acid and toluene
- Carbon tetrachloride and methanol


Negative Deviation
In case of negative deviations from Raoult’s law, the intermolecular attractive forces between A-A and B-B are weaker than those between A-B, which decreases the escaping tendency of molecules for each component, leading to a decrease in vapour pressure.
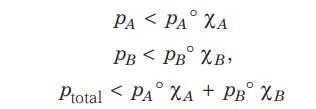
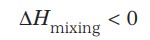
Example 1 - Mixture of phenol and aniline. In this case, the intermolecular hydrogen bonding between the phenolic proton and the lone pair on the nitrogen atom of aniline is stronger than the respective intermolecular hydrogen bonding between similar molecules.
Other examples -
- Water and hydrochloric acid (HCl)
- Acetone and aniline
- Chloroform and diethyl ether
- Chloroform and benzene
- Chloroform and acetone


Azeotropes (Introduction)
Definition - Some liquids, on mixing, form azeotropes, which are binary mixtures having the same composition in liquid and vapour phase and boil at a constant temperature.
In such cases, it is not possible to separate the components by fractional distillation.
There are two types of azeotropes -
- Minimum boiling azeotrope
- Maximum boiling azeotrope.


Maximum And Minimum Boiling Azeotrope
The solutions that show large negative deviation and the boiling point of which is more than either of the two pure components form a maximum boiling azeotrope at a specific composition.
Example - Nitric acid and water is an example of this class of azeotrope. This azeotrope has the approximate composition, 68% nitric acid and 32% water by mass, with a boiling point of 393.5 K.
Other examples - Water with HCl, Water with HCOOH, Chloroform with Acetone
_________________________________________________________________
The solutions that show large positive deviation and the boiling point of which is lesser than either of the two pure components form a minimum boiling azeotrope at a specific composition.
Example - Ethanol-water mixture (obtained by fermentation of sugars) on fractional distillation gives a solution containing approximately 95% by volume of ethanol. Once this composition, known as an azeotrope composition, has been achieved, the liquid and vapour have the same composition, and no further separation occurs.
Other examples - Chloroform with Ethanol


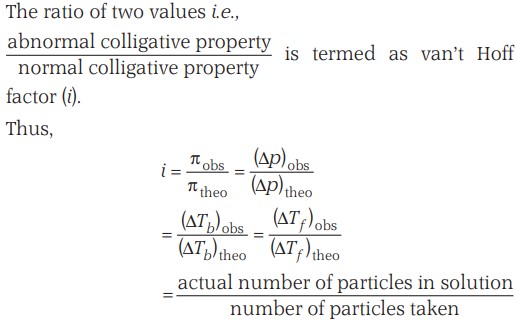
Colligative Properties (Introduction)
Definition - All the properties that depend on the number of solute particles irrespective of their nature relative to the total number of particles present in the solution are called colligative properties (colligative: from Latin: co means together, ligare means to bind).
Properties -
- Relative lowering of the vapour pressure of the solvent
- Depression of the freezing point of the solvent
- Elevation of the boiling point of the solvent
- Osmotic pressure of the solution.


Relative Lowering Of Vapour Pressure
When a non-volatile solute is added to a solvent, the vapour pressure is lowered. So, the vapour pressure of a solution (p) is always less than the vapour pressure of pure solvent (p°) at that temperature.
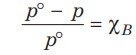
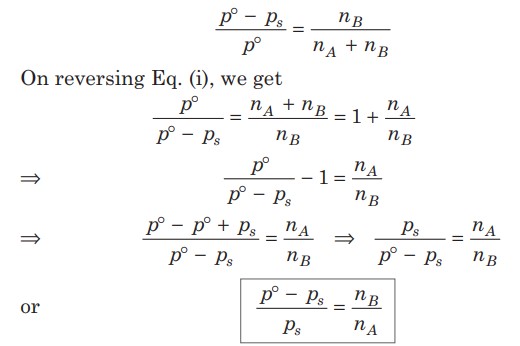
This expression is used to find the molecular weight of an unknown solute dissolved in a given solvent.


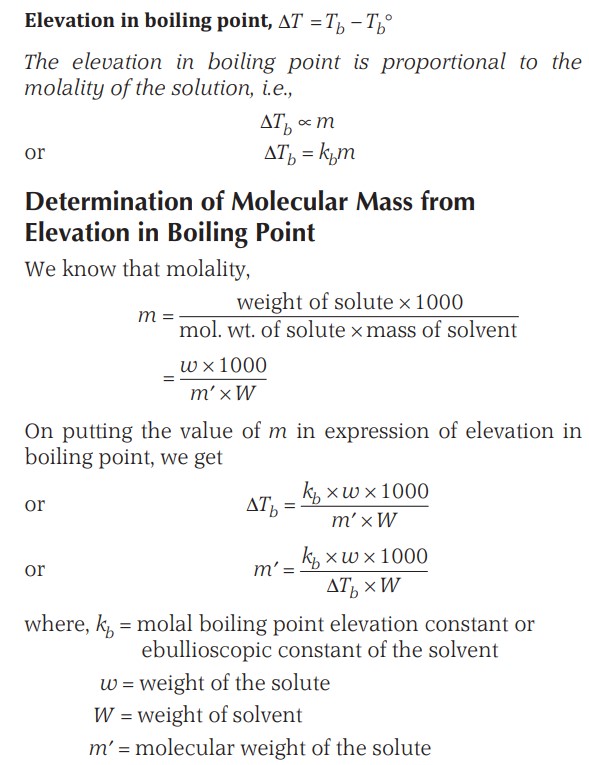
Elevation Of Boiling Point (Ebullioscopy)
As the vapour pressure of a solution is decreased due to the presence of a non-volatile solute, the solution boils at a higher temperature as compared to the pure solvent.
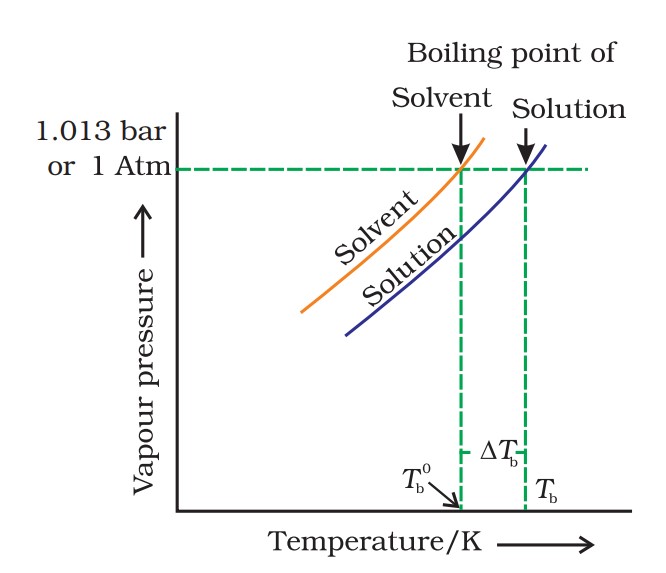
If Tb° is the boiling point of pure liquid and Tb is the boiling point of the solution, then,


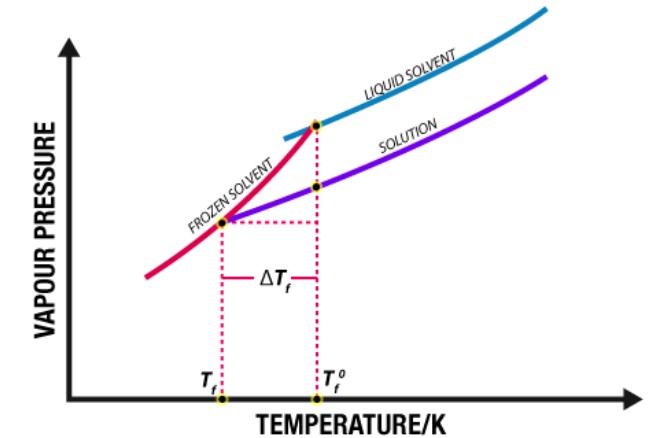
Depression Of Freezing Point
Since, the vapour pressure of a solvent is lowered by the addition of a non-volatile solute, the freezing point of the solution (Tf) is always lower than the freezing point of the pure solvent (Tf°) Therefore, depression in freezing point,
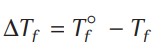
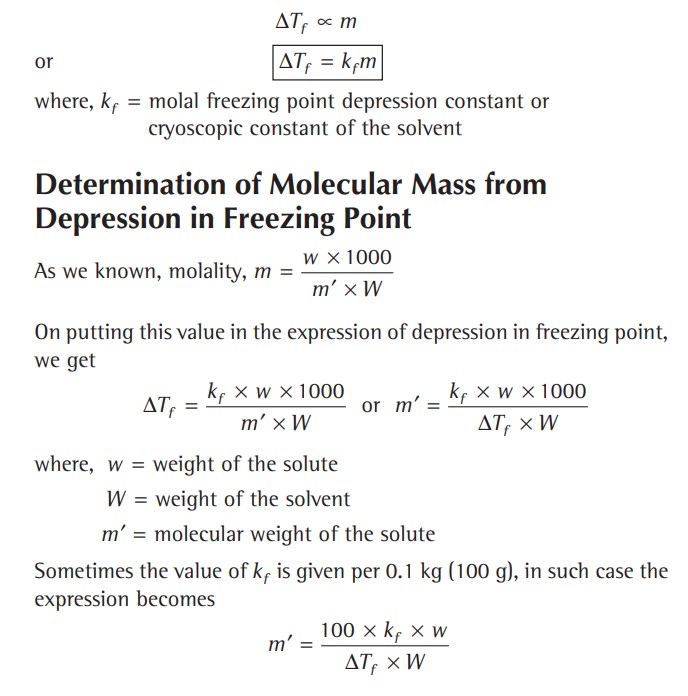
The depression in freezing point is proportional to the molality of the solution, i.e.,


Osmosis (Introduction)
Osmosis is the spontaneous flow of the solvent molecules through semipermeable membrane from a pure solvent to a solution or from a dilute to a concentrated solution.
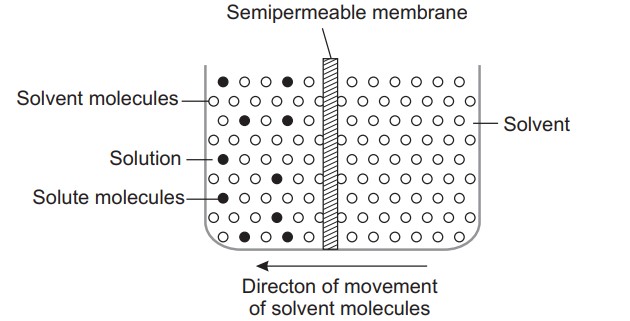
Semipermeable membranes are thin sheets, which allow the passage of only solvent molecules through them.
- Natural examples include - Egg membrane, goat’s bladder, cell membrane
- Artificial membranes include - calcium phosphate, copper ferrocyanide, freshly prepared silicates of iron, cobalt, nickel, etc.
- Cellophane and parchment papers are also used for the same purpose.
1. Osmotic pressure measurement provided a very good method of determination of molecular mass of polymers like proteins, etc., because a solution containing fewer particles is appreciable and also, as it is measured at room temperature, the method is particularly useful for biomolecules that are generally unstable at higher temperatures.
2. Osmosis plays a vital role in biology also. Some examples of it are -
- Movement of water from roots to the top of plants takes place via osmosis.
- A 0.91% solution of pure NaCl is isotonic with human red blood red cells (RBCs). On placing RBCs in hypotonic solution, they will swell and even burst (Edema). On placing RBCs in hypertonic solution, they shink due to plasmolysis.
- The use of salt and sugar as preservatives in pickels and jams has its basis in preventing growth of fungi and bacteria by osmosis
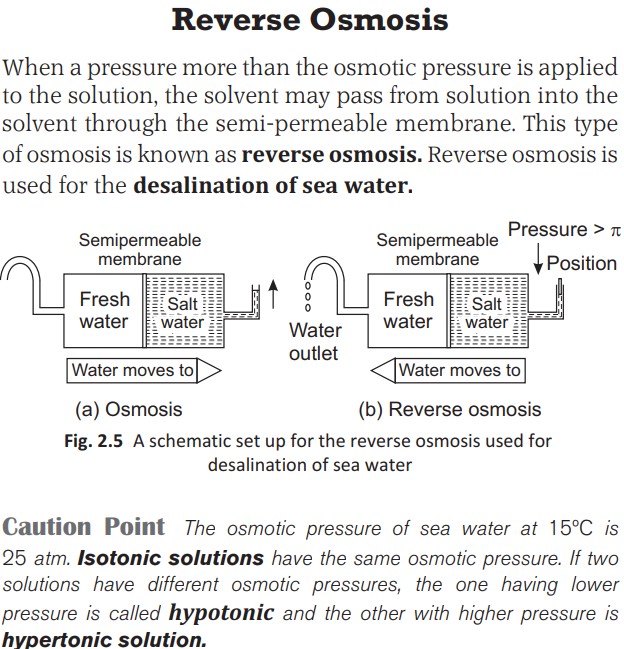
Reverse Osmosis is used in Dialysis, Desalination of sea water etc.


Osmotic Pressure And Reverse Osmosis
Osmotic Pressure is the external pressure that must be applied on the solution in order to stop the flow of the solvent into the solution through semipermeable membrane.
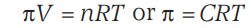
π - Osmotic pressure
C - Concentration
R - Gas constant
T - Temperature


 beeTokens
beeTokens