Classification
The halogen derivatives (both aliphatic and aromatic) can also be classified as mono, di, tri and tetra halogen derivatives depending upon the number of halogen atoms present in the molecule.

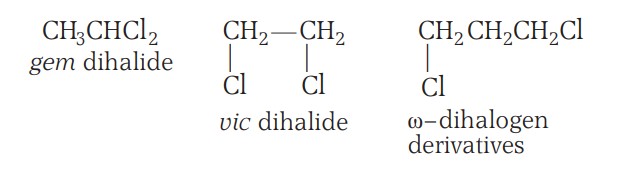
If both the halogen atoms are present at the same carbon atom, it is called geminal (gem-) dihalides. If present at adjacent carbon atoms, it is called vicinal (vic) dihalides.


Classification Continuation
(a) Alkyl halides or haloalkanes (R—X)
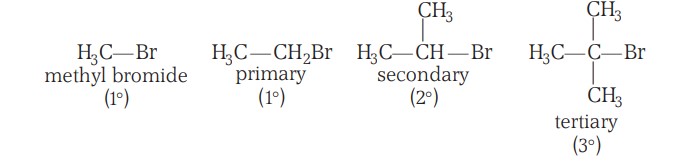
(b) Allylic halides - These are the compounds in which the halogen atom is bonded to an sp3-sp3-hybridised carbon atom adjacent to a carbon-carbon double bond (C=C), i.e. to an allylic carbon.
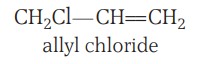
(c) Benzylic halides - These are the compounds in which the halogen atom is bonded to an sp3-hybridised carbon atom attached to an aromatic ring.
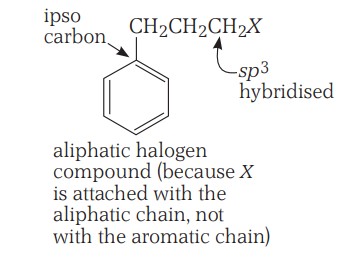


Classification Continuation.
(a) Vinylic halides - These are the compounds in which the halogen atom is bonded to a sp2-hybridised carbon atom of a carbon-carbon double bond

(b) Aryl halides - These are the compounds in which the halogen atom is directly bonded to the sp2-hybridised carbon atom of an aromatic ring.



Preparation - From Alcohols

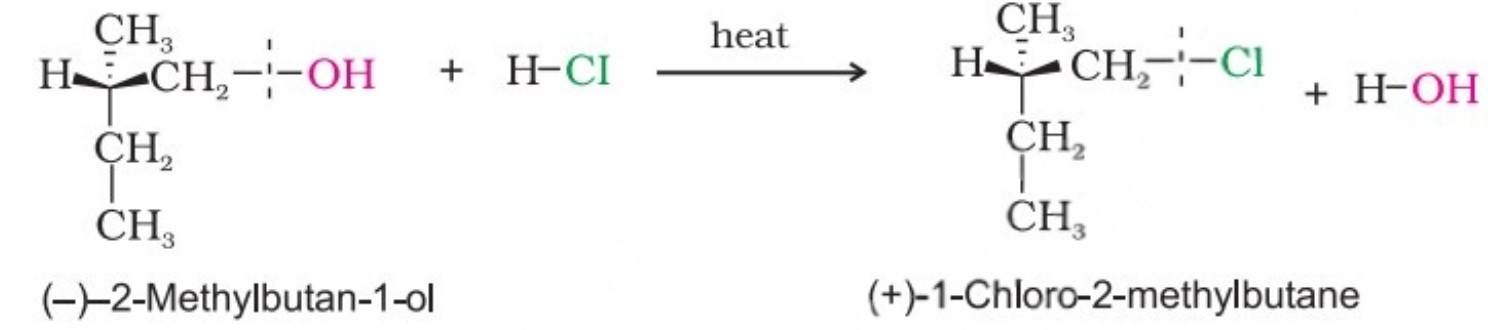
The hydroxyl group of an alcohol is replaced by halogen on reaction with concentrated halogen acids, phosphorus halides or thionyl chloride. Thionyl chloride is preferred because in this reaction alkyl halide is formed along with gases SO2 and HCl. The two gaseous products are escapable, hence, the reaction gives pure alkyl halides.



Preparation - From Hydrocarbons (I)
(I) From alkanes by free radical halogenation
Free radical chlorination or bromination of alkanes gives a complex mixture of isomeric mono- and polyhaloalkanes, which is difficult to separate as pure compounds. Consequently, the yield of any single compound is low.
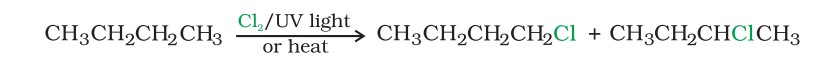


Preparation - From Hydrocarbons (II)
(II) From alkenes
(i) Addition of hydrogen halides: An alkene is converted to the corresponding alkyl halide by reaction with hydrogen chloride, hydrogen bromide or hydrogen iodide.
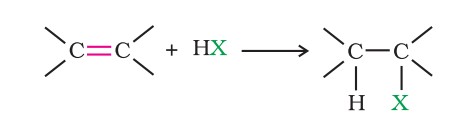
Propene yields two products, however, only one predominates as per Markovnikov’s rule.
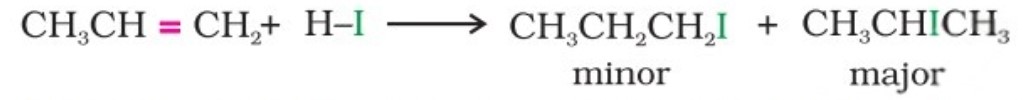
(ii) Addition of halogens: In the laboratory, the addition of bromine in Carbon Tetrachloride to an alkene, resulting in the discharge of reddish brown colour of bromine, constitutes an important method for the detection of a double bond in a molecule.
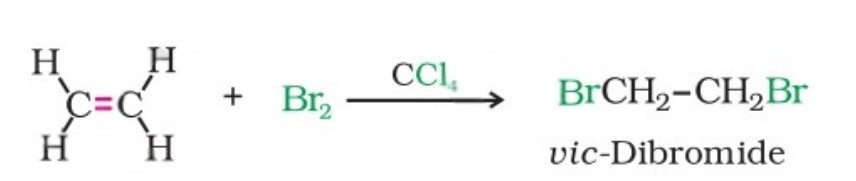
The addition results in the synthesis of vic-dibromides, which are colourless.


Preparation - From Hydrocarbons (III)
Halogen Exchange
(i) Finkelstein reaction - Alkyl iodides are often prepared by the reaction of alkyl chlorides/ bromides with NaI in dry acetone.

(ii) Swarts reaction - The synthesis of alkyl fluorides is best accomplished by heating an alkyl chloride/bromide in the presence of a metallic fluoride.

Examples of some metallic fluorides -
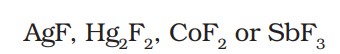


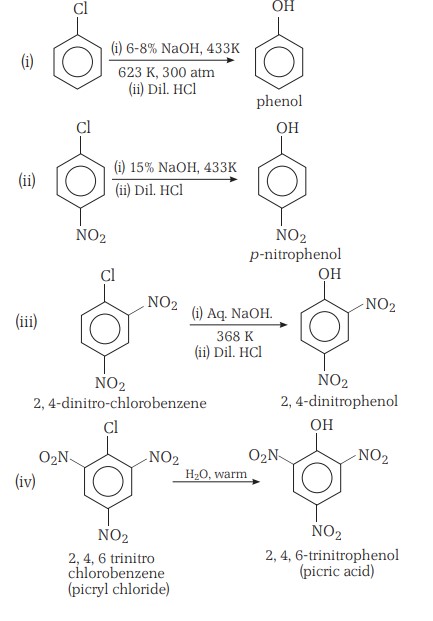
Preparation Of Haloarenes (I)
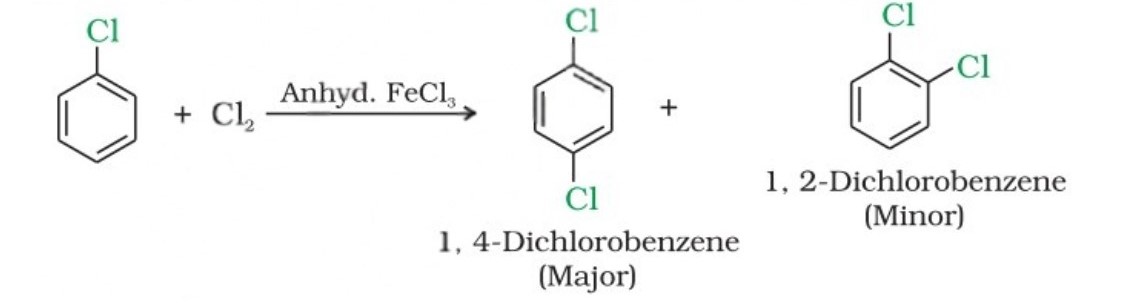
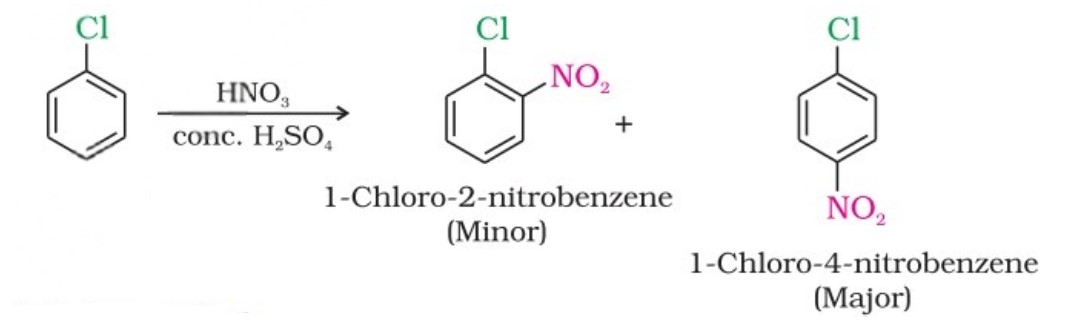
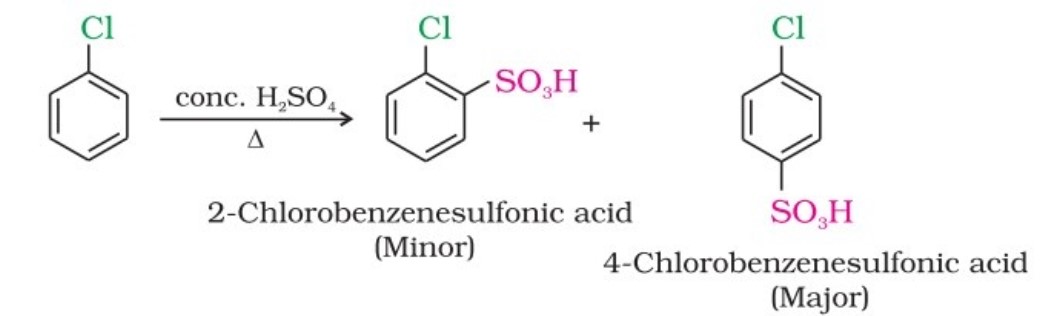
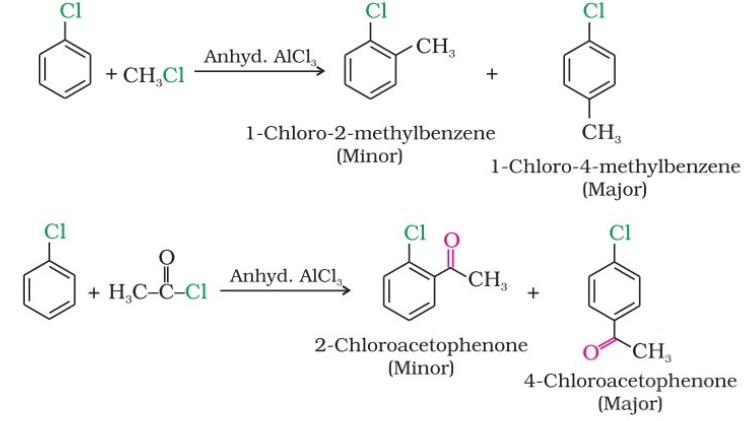
(i) From hydrocarbons by electrophilic substitution
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine, respectively, in the presence of Lewis acid catalysts like iron or iron(III) chloride.
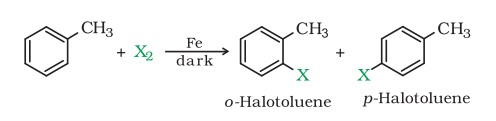
- The ortho and para isomers can be easily separated due to the large difference in their melting points.
- Reactions with iodine are reversible and require the presence of an oxidising agent (HNO3 , HIO4 ) to oxidise the HI formed during iodination.
- Fluoro compounds are not prepared by this method due to the high reactivity of fluorine.


Preparation Of Haloarenes (II)
(ii) From amines by Sandmeyer’s reaction
Mixing the solution of freshly prepared diazonium salt with cuprous chloride or cuprous bromide results in the replacement of the diazonium group by –Cl or –Br.



Preparation Of Haloarenes (III)
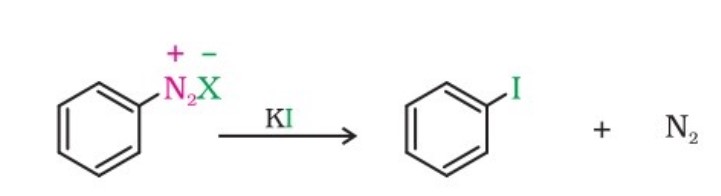
(iii) Replacement of the diazonium group by Iodine
This does not require the presence of cuprous halide and is done simply by shaking the diazonium salt with potassium iodide. It is not a Sandmeyer's reaction.


Physical Properties Of Halo Compounds
- Alkyl halides are colourless when pure. However, bromides and iodides develop colour when exposed to light. Many volatile halogen compounds have a sweet smell.
- Density - Bromo, iodo and polychloro derivatives of hydrocarbons are heavier than water. The density increases with an increase in the number of carbon atoms, halogen atoms and atomic mass of the halogen atoms
- Solubility - The haloalkanes are very slightly soluble in water. In order to dissolve haloalkane in water, energy is required to overcome the attractions between the haloalkane molecules and break the hydrogen bonds between water molecules. Thus, the solubility of haloalkanes in water is low. However, haloalkanes tend to dissolve in organic solvents because the new intermolecular attractions between haloalkanes and solvent molecules have much the same strength as the ones being broken in the separate haloalkane and solvent molecules.


Physical Properties Of Halo Compounds.
Boiling Point orders -
- For the same alkyl group, the BP of alkyl halides decrease in the order - RI> RBr> RCl> RF.
- The boiling points of isomeric haloalkanes decrease with an increase in branching.

- Para-isomers are high melting as compared to their ortho and meta-isomers. It is due to the symmetry of para-isomers that fit in the crystal lattice better as compared to ortho and meta-isomers.
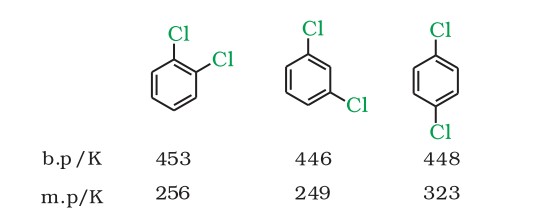
Melting Point orders -
For dihalobenzenes, the order is - Para > Ortho > Meta


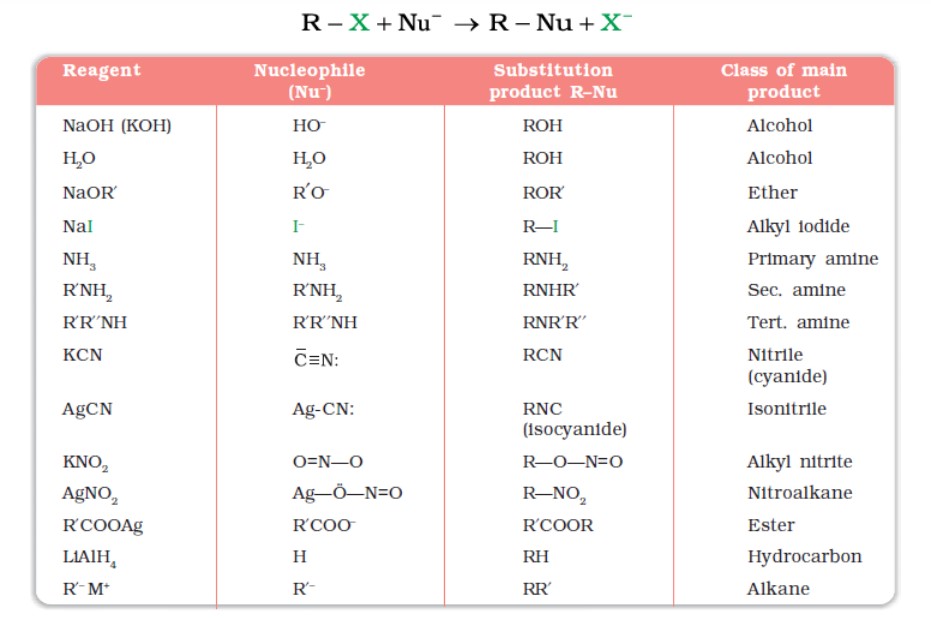
Nucleophilic Substitution Reactions
A substitution reaction takes place, and the halogen atom, called the leaving group, departs as a halide ion. Since a nucleophile initiates the substitution reaction, it is referred to as a nucleophilic substitution reaction.


Ambident Nucleophiles
An ambident nucleophile is a molecule or ion that can act as a nucleophile, donating an electron pair to form a bond, from two or more different atoms within the same molecule.
- Groups like cyanides and nitrites possess two nucleophilic centres. The cyanide group is a hybrid of two contributing structures and can act as a nucleophile in two different ways, i.e., linking through a carbon atom, resulting in alkyl cyanides and through a nitrogen atom, leading to isocyanides.
IMPORTANT NOTE -
KCN is predominantly ionic and provides cyanide ions in solution. Although both carbon and nitrogen atoms are in a position to donate electron pairs, the attack takes place mainly through carbon atom and not through nitrogen atom since C—C bond is more stable than C—N bond.
However, AgCN is mainly covalent in nature and nitrogen is free to donate electron pair, forming isocyanide as the main product.
- Similarly, nitrite ion also represents an ambident nucleophile with two different points of linkage. The linkage through oxygen results in alkyl nitrites, while through the nitrogen atom, it leads to nitroalkanes.



Substitution Nucleophilic Bimolecular (SN2)
- Follows a second-order kinetics, i.e., the rate depends upon the concentration of both reactants.
- The reaction takes place in a single step, and no intermediate is formed.
- The order of reactivity followed is: Primary halide > Secondary halide > Tertiary halide (Steric Hinderance)
- Takes place in Polar Aprotic Solvents (Polar, but no Hydrogen)
- Leads to Inversion of Configuration
- There is a Transition Unstable state that cannot be isolated.
Example -



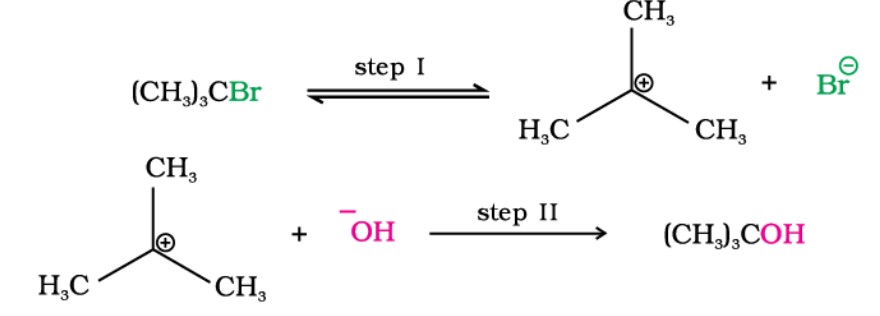
Substitution Nucleophilic Unimolecular (SN1)
- Generally follows a first-order kinetics, i.e., the rate depends upon the concentration of both reactants. However, Step 1 is slowest and the rate-determining step.
- The reaction takes place in two steps, and an intermediate is formed.
- The order of reactivity followed is: Tertiary halide > Secondary halide > Primary halide (Carbocation stability)
- Takes place in Polar Protic Solvents (Polar with Hydrogen, like ROH, Water)
- Leads to Racemisation (50% inversion + 50% retention)
Example -


Stereochemical Aspect (I)
(i) Optical activity:
The plane of plane-polarised light produced by passing ordinary light through a Nicol prism is rotated when it is passed through the solutions of certain compounds. Such compounds are called optically active compounds.
- If the compound rotates the plane of plane polarised light to the right, i.e., clockwise direction, it is called dextrorotatory or the d-form and is indicated by placing a positive (+) sign before the degree of rotation.
- If the light is rotated towards left (anticlockwise direction), the compound is said to be laevo-rotatory or the l-form and a negative (–) sign is placed before the degree of rotation.
Such (+) and (–) isomers of a compound are called optical isomers, and the phenomenon is termed optical isomerism.


Stereochemical Aspect (II)
(ii) Chirality and enantiomers:
The objects that are non-superimposable on their mirror image (like a pair of hands) are said to be chiral, and this property is known as chirality.
In Butan-2-ol, the carbon atom is attached to 4 different groups, hence it is a chiral carbon.
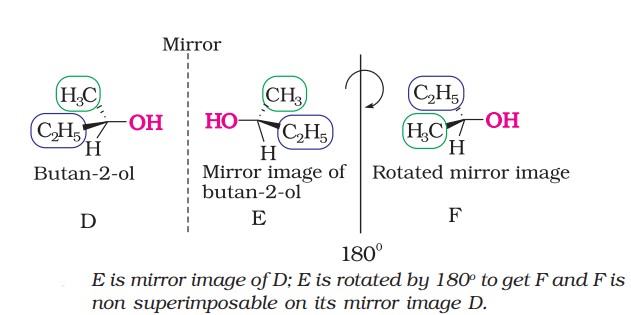
The stereoisomers related to each other as non-superimposable mirror images are called enantiomers.
Enantiomers possess identical physical properties, namely, melting point, boiling point, refractive index, etc. They only differ concerning the rotation of plane-polarised light. If one of the enantiomers is dextrorotatory, the other will be laevorotatory.


Racemisation
Racemic mixture or racemic modification : A mixture containing two enantiomers in equal proportions will have zero optical rotation, as the rotation due to one isomer will be cancelled by the rotation due to the other isomer.
A racemic mixture is represented by prefixing dl or (±) before the name, for example, (±) butan-2-ol. The process of conversion of an enantiomer into a racemic mixture is known as racemisation.


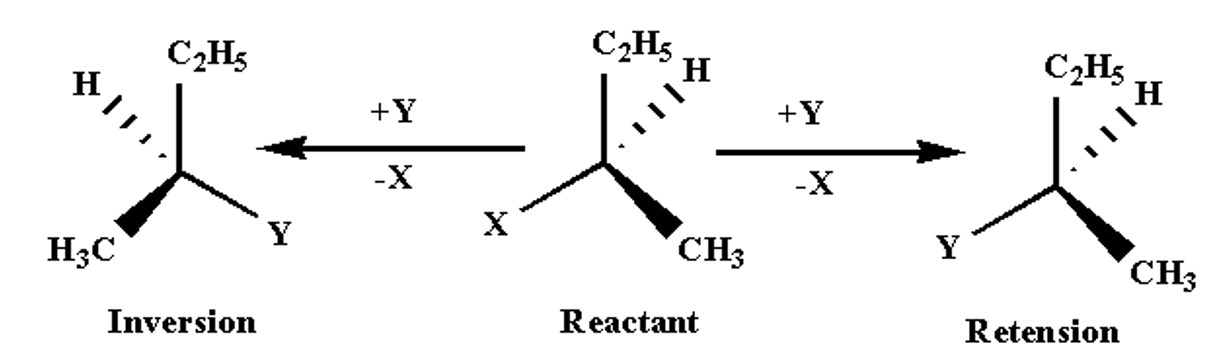
Retention And Inversion Of Configuration
Retention of configuration refers to the preservation of the spatial arrangement of bonds around an asymmetric centre during a chemical reaction or transformation.
Inversion of configuration is the reversal of the spatial arrangement of bonds to an asymmetric centre during a chemical reaction or transformation.
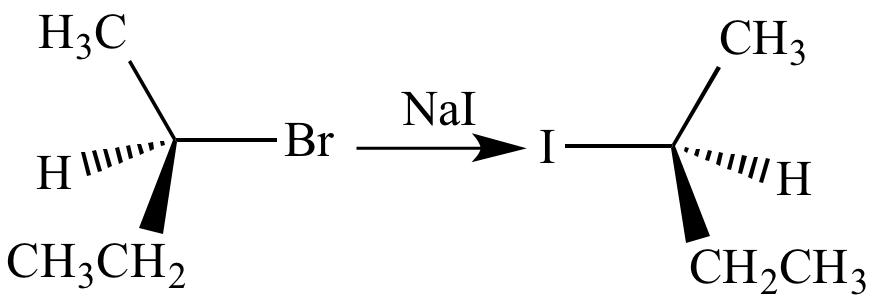


Elimination Reactions
When a haloalkane with a β-hydrogen atom is heated with an alcoholic solution of potassium hydroxide, there is elimination of a hydrogen atom from the β-carbon and a halogen atom from the α-carbon.
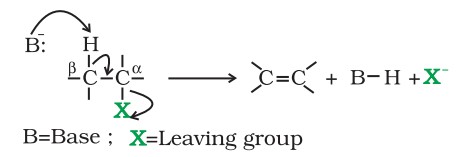
As a result, an alkene is formed as a product. Since the β-hydrogen atom is involved in elimination, it is often referred to as β-elimination.


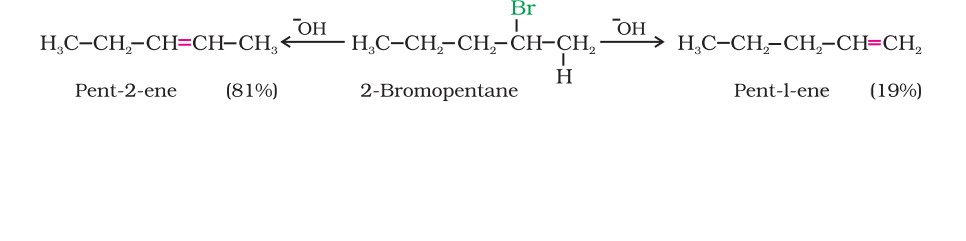
Saytzeff's Rule
If there is a possibility of the formation of more than one alkene due to the availability of more than one β-hydrogen atom, usually one alkene is formed as the major product.
Saytzeff's Rule - “In dehydrohalogenation reactions, the preferred product is that alkene which has the greater number of alkyl groups attached to the doubly bonded carbon atoms."
Thus, here, 2-bromopentane gives pent-2-ene as the major product.


Reaction With Metals (I)
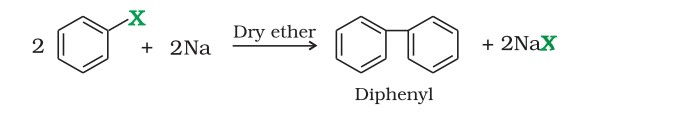
Wurtz-Fittig reaction - A mixture of an alkyl halide and aryl halide gives an alkylarene when treated with sodium in dry ether.

Wurtz-Fittig reaction - Aryl halides also give analogous compounds when treated with sodium in dry ether, in which two aryl groups are joined together.


Reaction With Metals (II)
Grignard Reagent - The reagents that are obtained by the reaction of haloalkanes with magnesium metal in dry ether.

Grignard reagents are highly reactive and react with any source of proton to give hydrocarbons.

It is therefore necessary to avoid even traces of moisture from a Grignard reagent. That is why the reaction is carried out in dry ether. On the other hand, this could be considered as one of the methods for converting halides to hydrocarbons.


Nucleophilic Substitution Reactions (I)
Aryl halides are extremely less reactive towards nucleophilic substitution reactions due to the following reasons:
(i) Resonance effect: C—Cl bond acquires a partial double bond character due to resonance. As a result, the bond cleavage in haloarene is difficult than haloalkane and therefore, they are less reactive towards the reaction.
(ii) Difference in hybridisation of carbon atom in C—X bond: The sp2 hybridised carbon with a greater s-character is more electronegative and can hold the electron pair of C—X bond more tightly than the sp3-hybridised carbon in haloalkane with less s-character. Since it is difficult to break a shorter bond than a longer bond, haloarenes are less reactive than haloalkanes towards nucleophilic substitution reactions.
(iii) Instability of phenyl cation: In case of haloarenes, the phenyl cation formed as a result of self-ionisation will not be stabilised by resonance and therefore, the SN1 mechanism is ruled out.
(iv) Because of the possible repulsion, it is less likely for the electron-rich nucleophile to approach electron-rich arenes.


Poly-halogen Compounds (I)
(i) Dichloromethane (Methylene chloride)
- Used as a solvent as a paint remover, as a propellant in aerosols, and as a process solvent in the manufacture of drugs.
- It is also used as a metal cleaning and finishing solvent. Methylene chloride harms the human central nervous system.
- Exposure to lower levels of methylene chloride in air can lead to slightly impaired hearing and vision, dizziness, nausea, tingling and numbness in the fingers and toes. In humans, direct skin contact with methylene chloride causes intense burning and mild redness of the skin. Direct contact with the eyes can burn the cornea.
(ii) Trichloromethane (Chloroform)
- Employed as a solvent for fats, alkaloids, iodine and other substances. The major use of chloroform today is in the production of the freon refrigerant R-22.
- It was once used as a general anaesthetic in surgery but has been replaced by less toxic, safer anaesthetics, such as ether. As might be expected from its use as an anaesthetic, inhaling chloroform vapours depresses the central nervous system.
- Chronic chloroform exposure may cause damage to the liver (where chloroform is metabolised to phosgene) and to the kidneys.
- Chloroform is slowly oxidised by air in the presence of light to an extremely poisonous gas, carbonyl chloride, also known as phosgene. It is therefore stored in closed dark coloured bottles completely filled so that air is kept out.
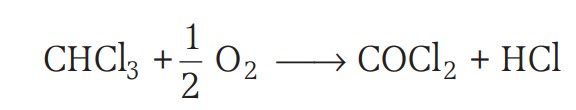
(iii)Triiodomethane (Iodoform)
It was used earlier as an antiseptic, but the antiseptic properties are due to the liberation of free iodine and not due to iodoform itself.


Poly-halogen Compounds (II)
(iv) Tetrachloromethane (Carbon tetrachloride)
- It is produced in large quantities for use in the manufacture of refrigerants and propellants for aerosol cans. It is also used as a feedstock in the synthesis of chlorofluorocarbons and other chemicals, pharmaceutical manufacturing, and general solvent use. The most common effects of use are dizziness, lightheadedness, nausea and vomiting, which can cause permanent damage to nerve cells.
- When carbon tetrachloride is released into the air, it rises to the atmosphere and depletes the ozone layer.
(v) Freons
- They are extremely stable, unreactive, non-toxic, noncorrosive and easily liquefiable gases.
- Freon 12 (CCl2F2 ) is one of the most common freons in industrial use. It is manufactured from tetrachloromethane by the Swarts reaction. These are usually produced for aerosol propellants, refrigeration and air conditioning purposes.
(vi) p,p’-Dichlorodiphenyltrichloroethane(DDT)
- The use of DDT increased enormously on a worldwide basis after World War II, primarily because of its effectiveness against the mosquito that spreads malaria and the lice that carry typhus. However, problems related to the extensive use of DDT began to appear in the late 1940s.
- Many species of insects developed resistance to DDT, and it was also discovered to have a high toxicity towards fish.
- The chemical stability of DDT and its fat solubility compounded the problem. DDT is not metabolised very rapidly by animals; instead, it is deposited and stored in the fatty tissues. If ingestion continues at a steady rate, DDT builds up within the animal over time.


 beeTokens
beeTokens