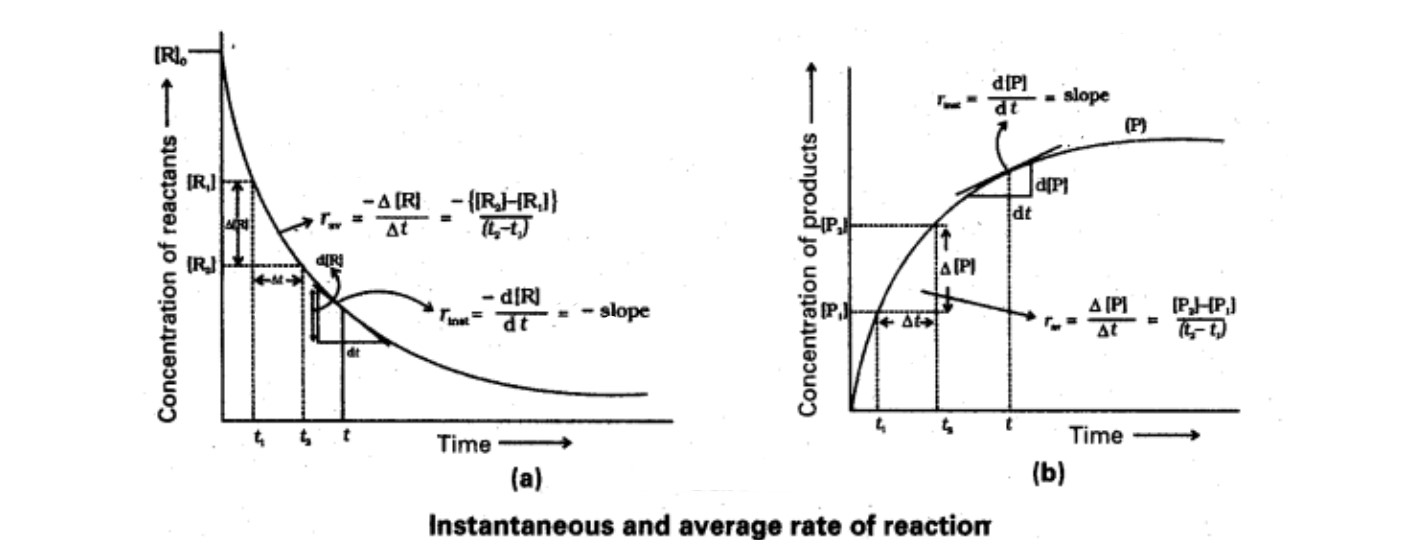
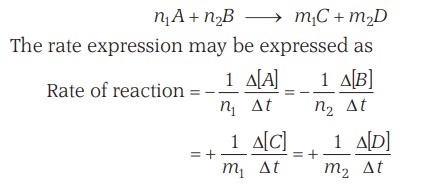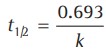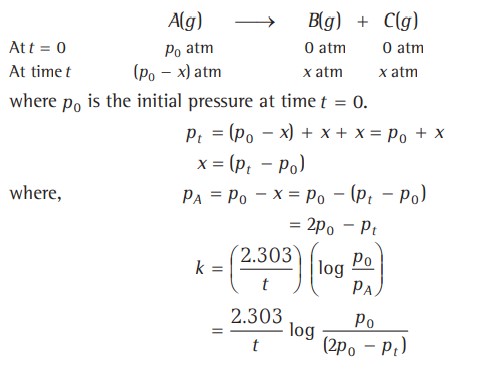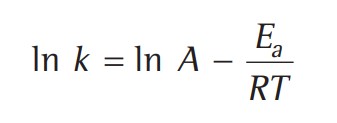Instantaneous And Average Rate Of A Reaction
The rate of reaction at any particular instant of time is known as the instantaneous rate of reaction. It is equal to the small change in concentration (dx) in a small interval of time (dt).
The average rate of a reaction is defined as the rate of change of concentration per unit time. It is calculated by dividing the total change in concentration of any one of the reactants or products by the total time taken to do so.


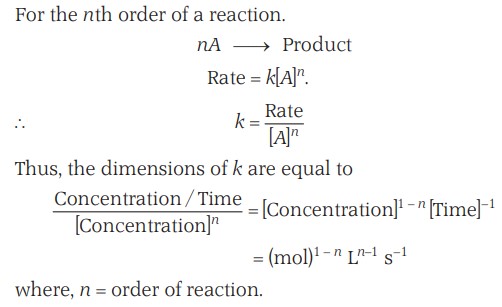
Units Of Rate Of Reaction
The rate of reaction has the units of concentration or molarity divided by time. Therefore, the units of rate of reaction may be expressed as (mol/L sec) or (mol/L min), etc.
However, in gaseous reactions, when the concentration of gases is expressed in terms of their partial pressures, then the units of the rate equation will be atm/sec.


Factors Affecting The Rate Of Reaction
The factors which affect the rate of reaction are as follows -
(a) Concentration of Reactants - An increase in the concentration of reactants increases the reaction rate.
(b) Nature of Reactants - The nature of bonds in a molecule influences the rate of reaction at which it changes into products.
(c) Temperature - Reaction rates are normally favoured by an increase in temperature. For most of the reactions, the rate is doubled for every 10°C rise in temperature.
(d) Presence of Catalyst - A catalyst is a substance that alters the reaction rate but itself remains unchanged in amount and chemical composition at the end of the reaction. It helps provide an alternate pathway for faster reaction.
(e) Surface Area of Reactants - The rate of reaction increases as the surface area of the reactant increases.
(f) Presence of Light - Reaction rate normally becomes faster in the presence of light as it gives the necessary activation energy for starting the reaction.


Rate Law
Rate law is the expression in which reaction rate is given in terms of molar concentration of reactants with each term raised to some power, which may or may not be the same as the stoichiometric coefficient of the reacting species in a balanced chemical equation.
If we consider the reaction,



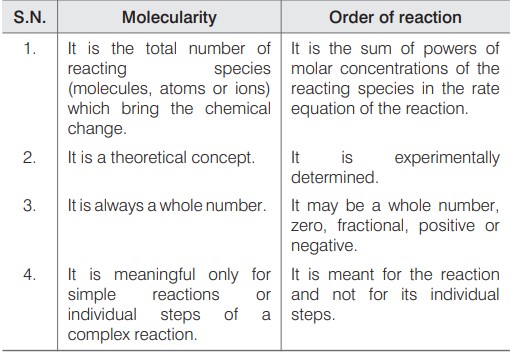
Order And Molecularity Of A Reaction
The sum of the powers of the concentration of the reactants in the rate law expression is called the order of that chemical reaction.
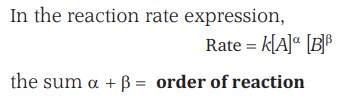
Molecularity can be defined as “The number of atoms or molecules that collide together at the same time for the reaction to occur”. Reactions are classified in terms of molecularity as unimolecular, bimolecular, and termolecular depending upon the number of molecules involved in the reaction.


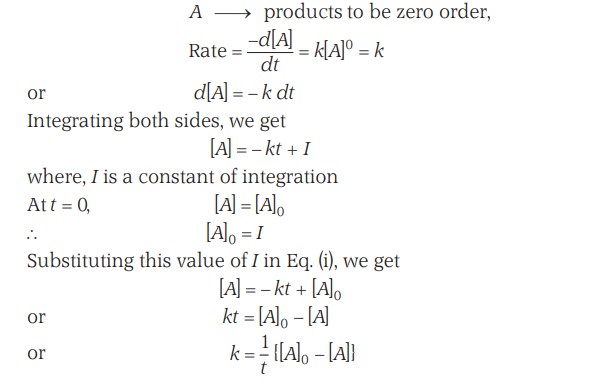
Zero Order Reactions - Half Life And Plot
The unit of rate constant for a zero-order reaction is mol/(L time)
The time required to reduce the initial concentration of the reactant to half of its initial value is called the half-life time or half-life period.
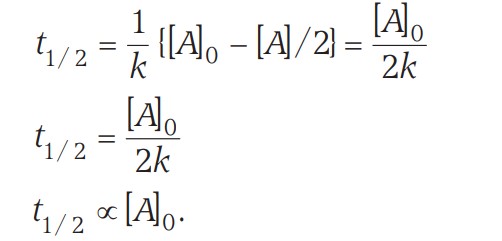
Thus, the half-life period of a zero-order reaction is directly proportional to the initial concentration.


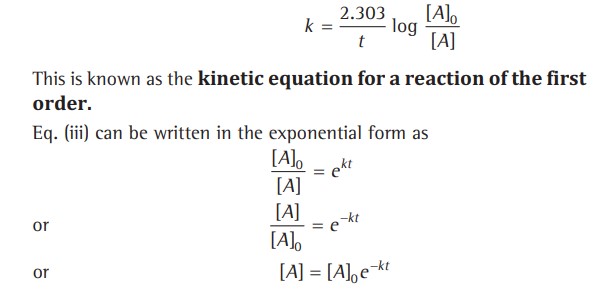
Pseudo First-Order Reactions.
Certain reactions that are expected to be of higher order follow the first-order kinetics and are said to be pseudo-first-order reactions.
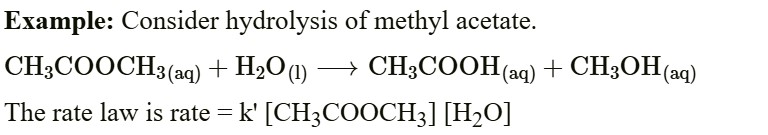
This reaction was expected to follow the second-order kinetics; however, it obeys the first-order kinetics.
Reason - Solvent water is present in such large excess that the change in its concentration is negligible compared to the initial one, or its concentration remains constant.


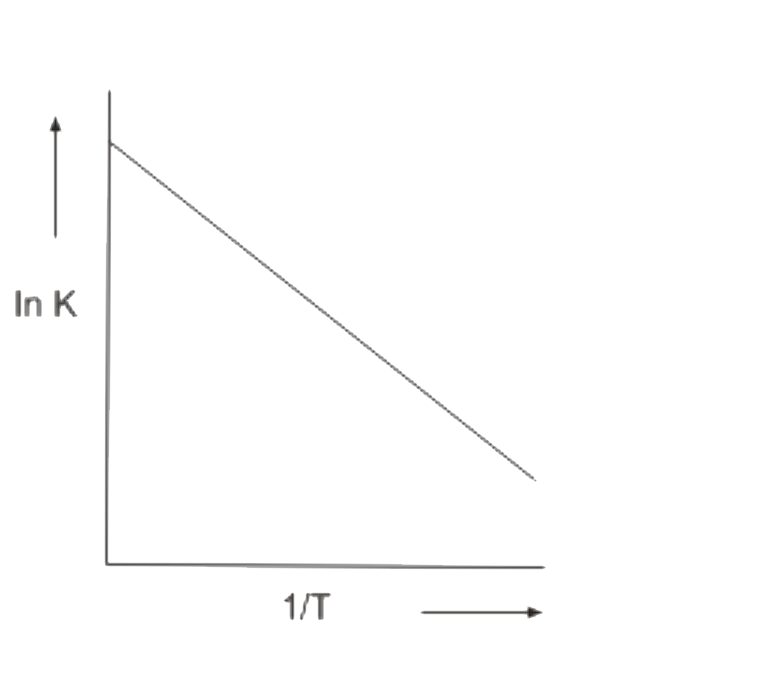
Temperature Dependence Of The Rate Of A Reaction
It has been found that for a chemical reaction with a rise in temperature by 10°, the rate constant is nearly doubled.
The temperature dependence of the rate of a chemical reaction can be accurately explained by the Arrhenius equation -
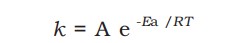
A - Arrhenius factor / Frequency factor / Pre-exponential factor.
R - Gas constant
Ea - activation energy measured in joules/mole


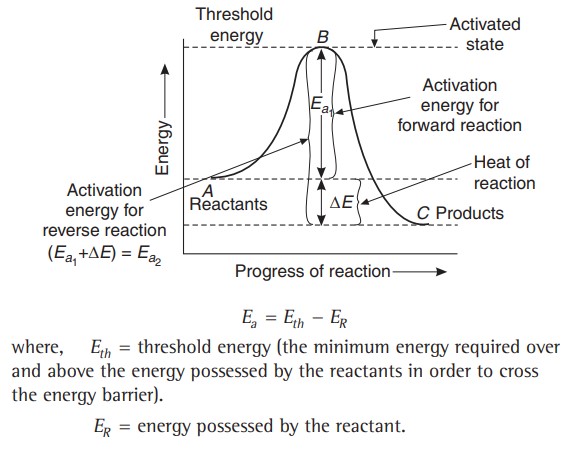
Activation Energy
The activation energy can also be defined as “The energy that activates passive or non-active molecules after its adsorption or minimum energy over the normal energy of molecules, which molecules must possess in order to form a product on collision".
The concept of activation energy as applied to chemical reactions can be explained by plotting energy against the progress of reaction, as shown below -


Distribution Curve
The peak of the curve corresponds to the most probable kinetic energy, i.e., kinetic energy of maximum fraction of molecules. There are decreasing number of molecules with energies higher or lower than this value.
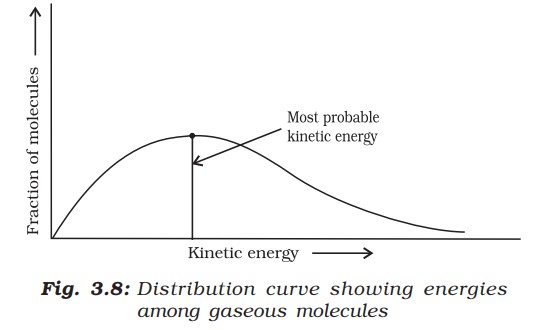
The distribution of kinetic energy may be described by plotting the fraction of molecules (Ne / Nt ) with a given kinetic energy (E) vs kinetic energy. Here, Ne is the number of molecules with energy E, and Nt is the total number of molecules.


Distribution Curve Continuation
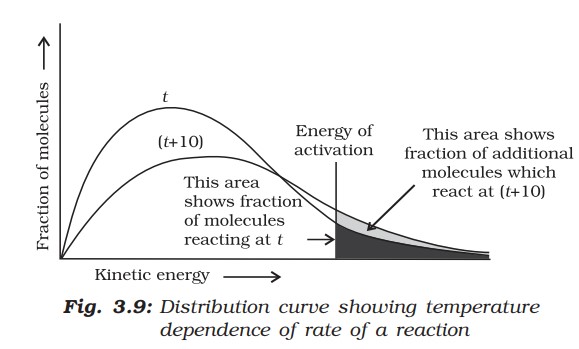
When the temperature is raised, the maximum of the curve moves to a higher energy value and the curve broadens out, i.e., spreads to the right, such that there is a greater proportion of molecules with much higher energies. The area under the curve must be constant since total probability must be one at all times.


Effect Of Catalyst
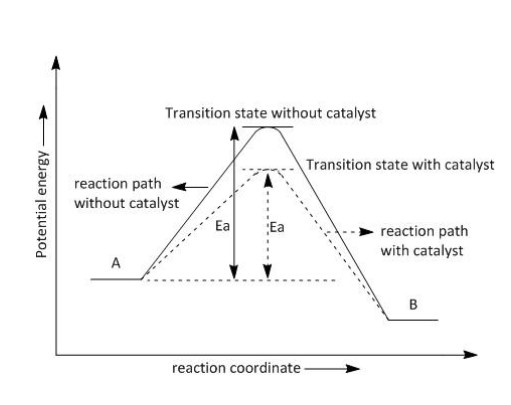
A catalyst is a substance that increases the rate of a reaction without itself undergoing any permanent chemical change.
- The catalyst provides an alternate pathway or reaction mechanism by reducing the activation energy between reactants and products and hence lowering the potential energy barrier.
- A small amount of the catalyst can catalyse a large amount of reactants.
- A catalyst does not alter the Gibbs energy of a reaction.


Collision Theory Of Chemical Reactions
The collision theory states that -
- If two molecules are to react together, they must collide with each other.
- The collision between all molecules does not lead to a chemical reaction.
- The collisions in which the molecules acquire energy greater than the activation energy result in a chemical reaction.
- The collisions in which molecules collide with sufficient kinetic energy (called threshold energy) and proper orientation, to facilitate the breaking of bonds between reacting species and the formation of new bonds to form products, are called as effective collisions.


Rate Expression
For a successful collision, however, it is also important that the molecules be oriented properly in space, along with their energy requirements.
Thus if -
- P - Orientation factor / Steric factor)
- Z - Collision frequency of reactants
Then the rate equation can be -
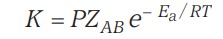


 beeTokens
beeTokens