Uses Of D And F Block Elements
- The steel in our cities is an alloy of iron, strengthened by transition metals like Cr, Mn, and Ni, whose electrons create powerful metallic bonds.
- The vibrant colours in pigments and dyes are born from the d-d electron transitions in compounds like TiO2.
- The industrial catalysts that produce our fertilisers (Fe) and clean fuels (Pt, Ni) depend on the ability of these metals to change oxidation states and form complexes.
- The nuclear energy that powers our world is harnessed from inner transition elements like Uranium.


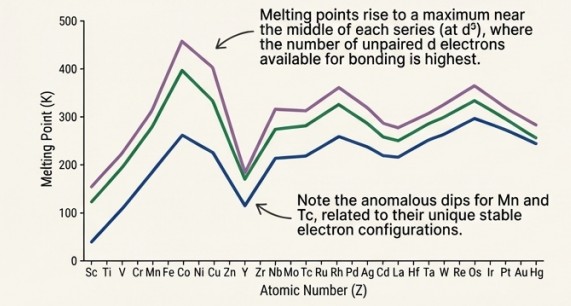
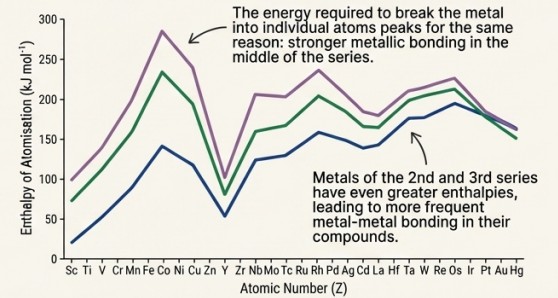
Enthalpies Of Atomisation
Mechanism:
Their strength comes from strong interatomic metallic bonding. This bonding involves not just the outer ns electrons, but also the numerous (n-1)d electrons. More shared electrons result in a stronger bond between atoms.
Visual Evidence:


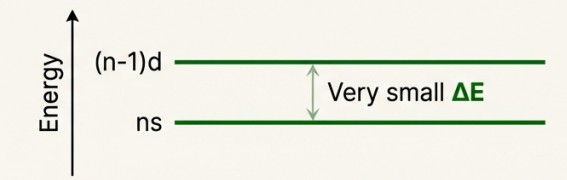
Variable Oxidation States
Mechanism:
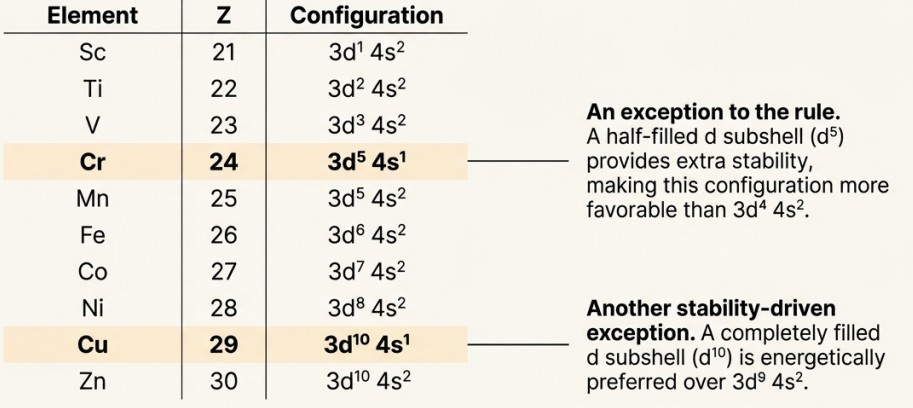
- Because the (n-1)d and ns orbitals are so close in energy, transition metals can lose a variable number of electrons to form ions.
- This creates a wide variety of oxidation states, often differing by a single unit (e.g., V2+, V3+). This is a key difference from non-transition elements.
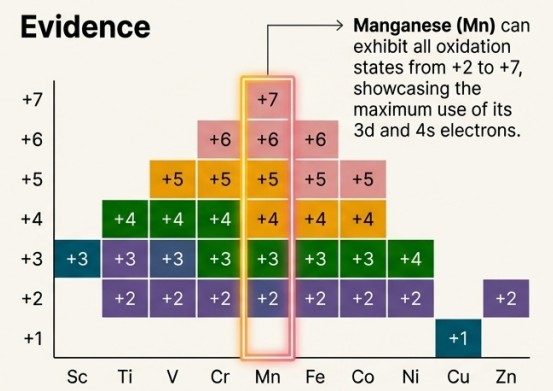
The greatest number of oxidation states occurs in or near the middle of the series, where there is a maximum number of electrons available to lose or share.


Transition Metals
According to IUPAC, transition metals are defined as elements that have an incomplete d subshell, either in their neutral atom or in their ions.
- There are mainly four series of the transition metals, 3d series (Sc to Zn), 4d series (Y to Cd), 5d series (La and Hf to Hg) and 6d series, which has Ac and elements from Rf to Cn.
- The two series of the inner transition metals, 4f (Ce to Lu) and 5f (Th to Lr), are known as lanthanoids and actinoids, respectively.


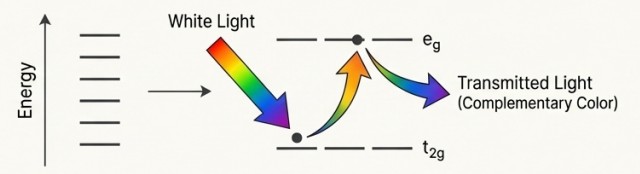
Color Of Transition Metals
Mechanism:
The color of transition metal ions is not magic; it's quantum mechanics in action.
- In a compound, the five d-orbitals split into lower and higher energy sets.
- When the ion has partially filled d-orbitals, an electron absorbs energy from visible light to "jump" to a higher-energy d-orbital. This is called a d-d transition.
- The light that isn't absorbed is what we see. The observed color is the complementary color to the light that was absorbed.


Magnetism From Unpaired Electrons
Phenomenon:
Many transition metal compounds are paramagnetic, meaning they are attracted to a magnetic field.
Mechanism:
Paramagnetism arises from the presence of unpaired electrons. Each unpaired electron has a magnetic moment due to its spin. The more unpaired electrons, the stronger the paramagnetic effect. Substances with no unpaired electrons are diamagnetic (repelled by magnetic fields).
Formula:



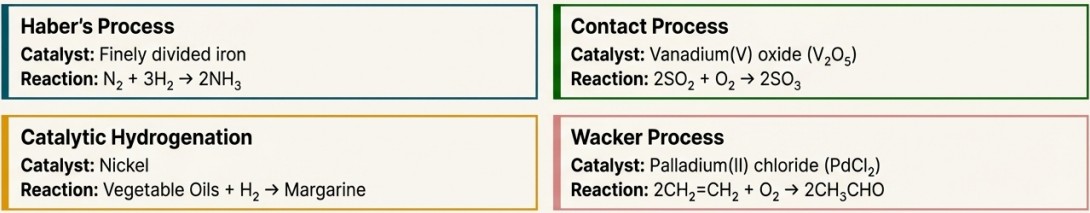
Catalytic Properties
- Multiple Oxidation States: They can easily accept and donate electrons, acting as a temporary shuttle. They can change oxidation states during a reaction and be regenerated at the end, ready for the next cycle.
- Complex Formation: They can use their d-orbitals to form temporary bonds with reactant molecules. This increases reactant concentration at the surface and weakens the existing bonds in the reactants, lowering the activation energy.


Potassium Dichromate
Potassium dichromate is a powerful oxidising agent, a role it plays because chromium can achieve a high +6 oxidation state.
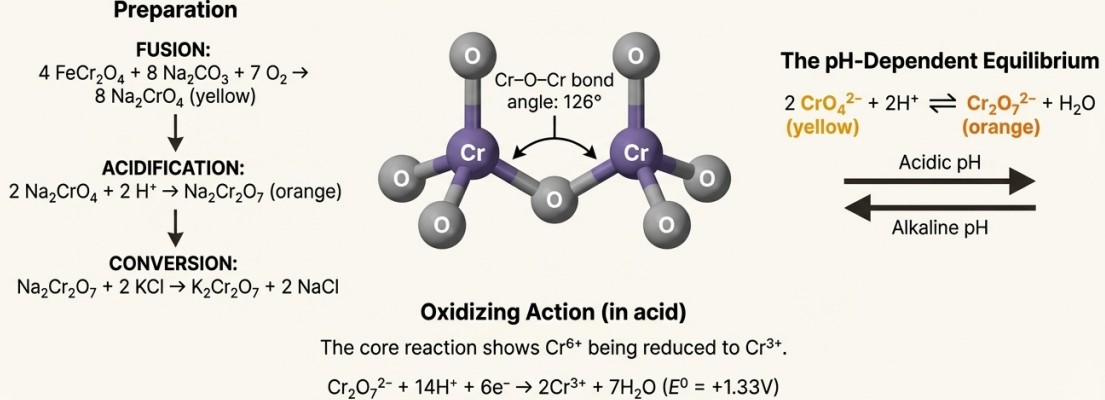
This high positive potential allows it to oxidise iodides to iodine, Fe2+ to Fe3+, and H₂S to sulfur.


Potassium Permanganate
In the intensely purple permanganate ion, manganese achieves its highest possible oxidation state: +7.

Applications:
- Volumetric Analysis: Titration of Fe2+ and oxalate ions.
- Organic Synthesis: Strong oxidant.
- Water Treatment/Disinfection: Due to its powerful oxidising properties.



F-Block Elements
Placed separately at the bottom of the periodic table are two series of elements where the inner 4f and 5f orbitals are progressively filled.
The Lanthanoids:
- The 14 elements following Lanthanum (La). They involve the filling of the 4f orbitals.
- General Symbol: Ln
- Known for their chemical similarity and one dominant oxidation state (+3).
The Actinoids:
- The 14 elements following Actinium (Ac). They involve the filling of the 5f orbitals.
- Known for their radioactivity and much more complex chemistry with a wider range of oxidation states.


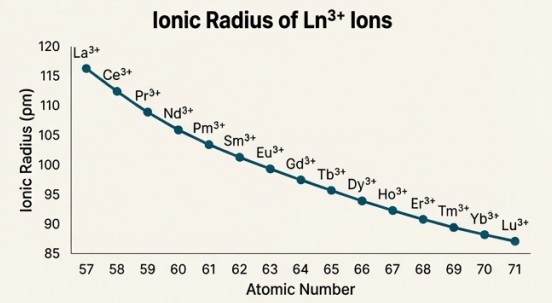
The Lanthanoid Contraction
Phenomenon:
There is a steady, gradual decrease in atomic and ionic radii across the lanthanoid series (from Cerium to Lutetium).
Mechanism:
As protons are added to the nucleus, electrons are added to the inner 4f orbitals. These 4f electrons provide very poor shielding of the increasing nuclear charge from the outer electrons. As a result, the outer electrons are pulled in more tightly, causing the atom/ion to shrink.
The cumulative effect of this contraction is so significant that the elements of the third transition series (5d) have almost identical atomic radii to their counterparts in the second transition series (4d).


The Actinoids
- Radioactivity: All actinoids are radioactive. The earlier members have long half-lives, but the later members have half-lives ranging from days to minutes, making their study extremely difficult.
- Wider Range of Oxidation States: Unlike lanthanoids (mostly +3), the early actinoids exhibit a broad range of oxidation states (e.g., Th (+4), U (+3 to +6), Np (+3 to +7)).
- Actinoid Contraction: A similar contraction in size occurs, but it is greater from element to element due to even poorer shielding by 5f electrons.
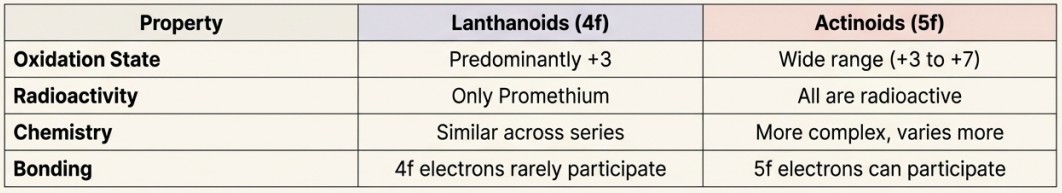
The Reason for Complexity: The 5f, 6d, and 7s orbitals are of comparable energy. The 5f orbitals are not as 'buried' as the 4f orbitals. This means that 5f electrons can, and do, participate in chemical bonding, leading to the greater variety of oxidation states.


 beeTokens
beeTokens