Introduction To Carbohydrates
Carbohydrates are naturally occurring organic substances. These are present in both plants and animals.
Scientifically, they may be defined as optically active polyhydroxy aldehydes or ketones or the compounds which produce such units on hydrolysis. Carbohydrates are also called saccharides.
Carbohydrates are classified based on their behaviour on hydrolysis, mainly-
- Monosaccharides (A carbohydrate that cannot be hydrolysed further to give simpler unit of polyhydroxy aldehyde or ketone. Example- glucose, fructose, ribose, etc.)
- Oligosaccharides (Carbohydrates that yield two to ten monosaccharide units, on hydrolysis. They are further classified as disaccharides, trisaccharides, tetrasaccharides, etc.)
- Polysaccharides (Carbohydrates which yield a large number of monosaccharide units on hydrolysis are called polysaccharides. Some common examples are starch, cellulose, glycogen, gums, etc)


Glucose Preparation
Preparation methods of Glucose include -
1. From Cane sugar (Sucrose) on acid hydrolysis in the presence of alcohol, gives a mixture of glucose and fructose.

2. From starch

3. From lactose or maltose
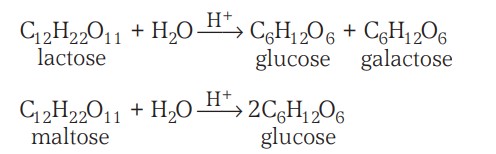


Structure Of Glucose
Glucose is an aldohexose and is also known as dextrose. It is the monomer of many of the larger carbohydrates, namely starch and cellulose.
Its structure can be determined through the following reactions -
1. Glucose reacts with hydroxylamine to form an oxime and adds a molecule of hydrogen cyanide to give cyanohydrin. These reactions confirm the presence of a carbonyl group.
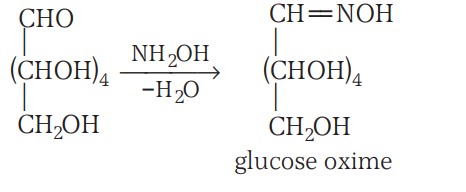
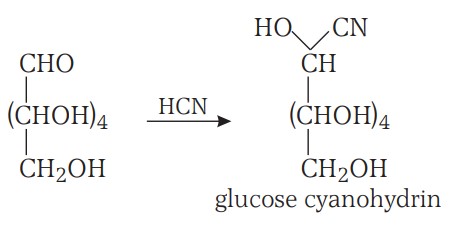


Structure Of Glucose Continuation.
2. On reduction with HI and red P, it gives a mixture of n-hexane and 2-iodohexane, suggesting that all six carbon atoms are linked in a straight chain.
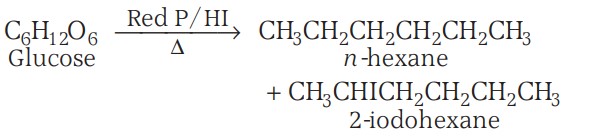
3. Glucose gets oxidised to six-carbon carboxylic acid (gluconic acid) on reaction with a mild oxidising agent like bromine water, indicating the presence of an aldehydic group.
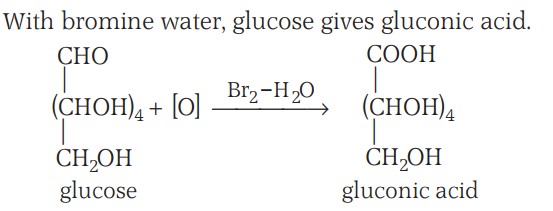


Structure Of Glucose Continuation
4. Acetylation of glucose with acetic anhydride gives glucose pentaacetate, which confirms the presence of five –OH groups attached to different carbon atoms.
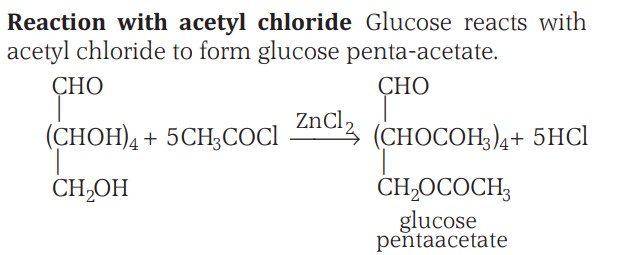
5. On oxidation with nitric acid, glucose as well as gluconic acid both yield a dicarboxylic acid, saccharic acid, indicating the presence of a primary alcoholic (–OH) group in glucose.
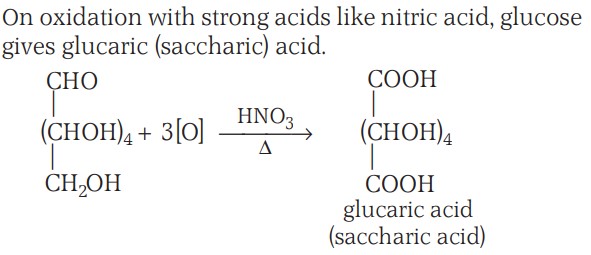


Glucose Structure
On the basis of point discussed, it was suggested that glucose has an open chain structure.

Evidence against open chain structure -
However, there are some evidences which do not support the open chain structure of glucose. These are -
1. Even though an aldehyde group is present, the glucose does not react with NaHSO4, 2,4-DNP and Grignard reagent.
2. Glucose does not respond to Schiff’s reagent test.
3. Glucose penta-acetate does not react with hydroxylamine. This indicates the absence of free —CHO group in glucose.
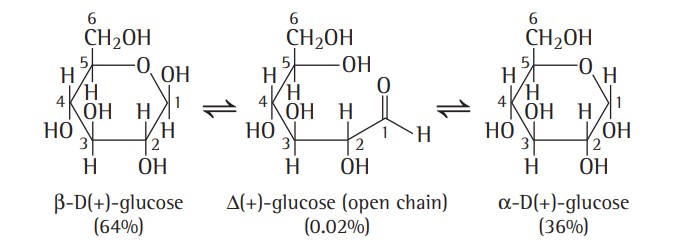
4. Glucose exists in two stereoisomeric forms i.e.,α and β anomers. α-glucose (mp 146°C) with specific rotation +111° is obtained by crystallising glucose from alcohol or acetic acid solution whereas β-glucose (mp 150°C) with specific rotation 19.2° is obtained by crystallising glucose form pyridine solution.
5. An aqueous solution of glucose shows mutarotation, i.e., if either of the two forms is dissolved in water and allowed to stand, the specific rotation of the solution changes gradually until a final value of +52.5° is reached.


Cyclic Structure Of Glucose
Hence, it was proposed that one of the —OH groups may add to the —CHO group to form a cyclic hemiacetal structure. According to Fischer, in this process, glucose forms a stable cyclic hemiacetal between the —CHO group and the —OH group of the fifth carbon atom in the pyranose structure.
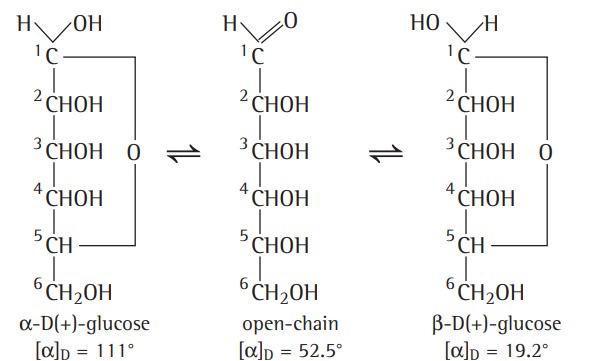
Haworth Projection formula -


Fructose
Fructose is an important ketohexose. It is obtained along with glucose by the hydrolysis of the disaccharide, sucrose. It is a natural monosaccharide found in fruits, honey and vegetables, also used as a sweetener.
It's structure includes - 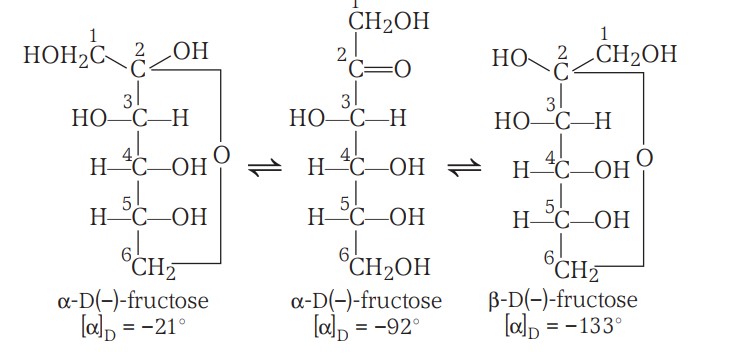
The Haworth Projection Formulae (name derived from Furan due to similarity in structure) -
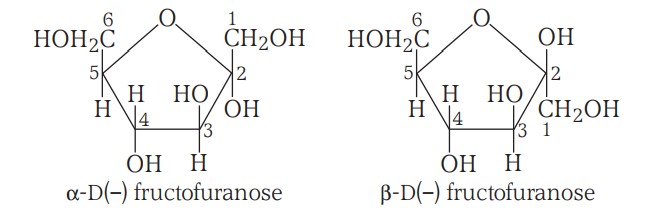


Sucrose
We already know that disaccharides on hydrolysis with dilute acids or enzymes, yield two molecules of either the same or different monosaccharides.
The two monosaccharides are joined together by an oxide linkage formed by the loss of a water molecule. Such a linkage between two monosaccharide units through an oxygen atom is called a glycosidic linkage.
Sucrose is formed using two monosaccharides, held together by a glycosidic linkage (between C1 of α-D-glucose and C2 of β-D-fructose)
Since the reducing groups of glucose and fructose are involved in glycosidic bond formation, sucrose is a non-reducing sugar.
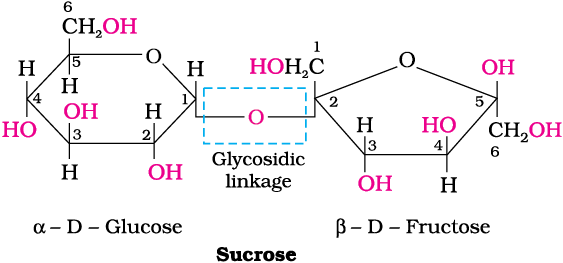
Sucrose is dextrorotatory but after hydrolysis gives dextrorotatory glucose and laevorotatory fructose. Since the laevorotation of fructose (–92.4°) is more than dextrorotation of glucose (+ 52.5°), the mixture is laevorotatory. Thus, hydrolysis of sucrose brings about a change in the sign of rotation and the product is named as invert sugar.


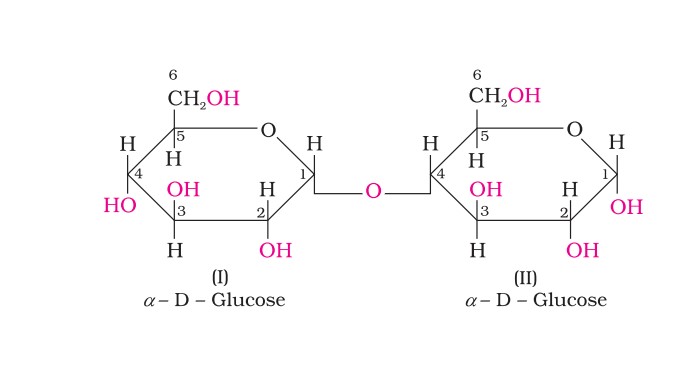
Maltose
Maltose is composed of two α-D-glucose units in which C1 of one glucose (I) is linked to C4 of another glucose unit (II). The free aldehyde group can be produced at C1 of the second glucose in solution, and it shows reducing properties so it is a reducing sugar.


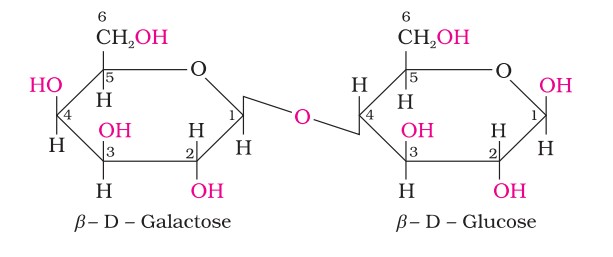
Lactose
Lactose is more commonly known as milk sugar since this disaccharide is found in milk. It is composed of β-D-galactose and β-D-glucose. The linkage is between C1 of galactose and C4 of glucose. A free aldehyde group may be produced at C-1 of the glucose unit, hence it is also a reducing sugar.


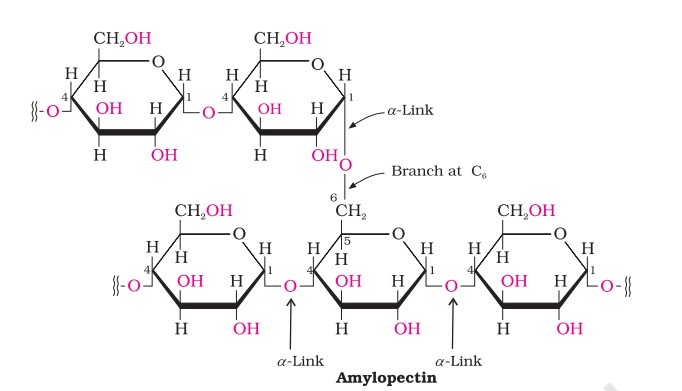
Starch - Amylopectin
Amylopectin is insoluble in water and constitutes about 80- 85% of starch. It is a branched chain polymer of α-D-glucose units in which the chain is formed by C1–C4 glycosidic linkage whereas branching occurs by C1–C6 glycosidic linkage.


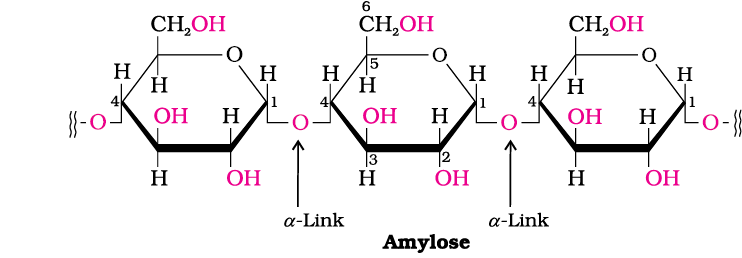
Starch - Amylose
Starch is the main storage polysaccharide of plants. It is the most important dietary source for human beings found in cereals, roots, tubers and some vegetables. It is a polymer of α-glucose and consists of two components— Amylose and Amylopectin.
Amylose is a water-soluble component constituting about 15-20% of starch. Chemically, amylose is a long unbranched chain with 200-1000 α-D-(+)-glucose units held together by C1–C4 glycosidic linkage.


Cellulose And Glycogen
Cellulose occurs exclusively in plants and it is the most abundant organic substance in plant kingdom. It is a predominant constituent of cell wall of plant cells. Cellulose is a straight-chain polysaccharide composed only of β-D-glucose units which are joined by glycosidic linkage between C1 of one glucose unit and C4 of the next glucose unit.
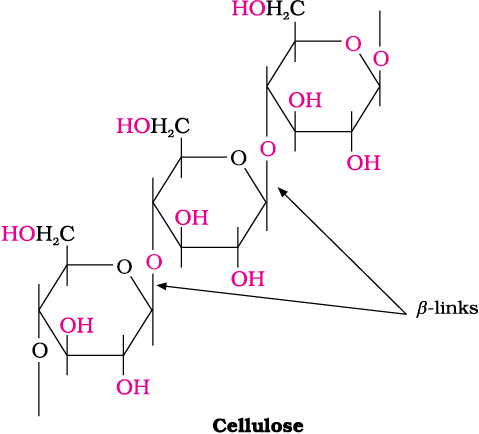
Glycogen: The carbohydrates are stored in the animal body as glycogen. It is also known as animal starch because its structure is similar to amylopectin and is rather more highly branched. It is present in liver, muscles and brain. When the body needs glucose, enzymes break down glycogen to glucose. It is also found in yeast and fungi.


Amino Acids
Amino acids contain amino (–NH2 ) and carboxyl (–COOH) functional groups. Depending upon the relative position of amino group with respect to carboxyl group, the amino acids can be classified. Only α-amino acids are obtained on the hydrolysis of proteins.
Essential and Non-Essential Amino Acids:
The amino acids, that can be synthesised in the body, are known as non-essential amino acids. Examples - Proline, Tyrosine, Glutamine etc.
On the other hand, those that cannot be synthesised in the body and must be obtained through diet are known as essential amino acids. Examples - Lysine, Histidine, Tryptophan etc.


Zwitter Ion Structure
Amino acids contain the —COOH group, which is acidic and the —NH2 group, which is basic. In the solid state, an amino acid ordinarily exists as a Zwitter ion or as a bipolar ion, formed by the transfer of a proton from a —COOH group to an —NH2 group.



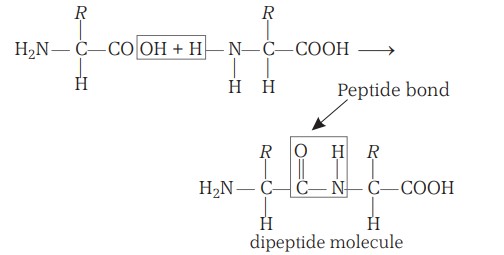
Peptide Bonds
Amino acids may be joined together by an amide linkage called a peptide linkage (CO—NH). A water molecule is always eliminated during the formation of a peptide linkage. Thereby, the molecule derived from two amino acids containing a single peptide linkage is called a dipeptide, and that derived from three amino acids is termed as a tripeptide. Peptides with more than 10 amino acid residues are called polypeptides.
A polypeptide with fewer a-amino acids may also be called a protein if it has a well defined conformation characteristic of a protein such as insulin (contains 51 amino-acids).
NOTE - Polypeptides are amphoteric in nature.


Fibrous And Globular Proteins
Fibrous Proteins - When the polypeptide chains run parallel and are held together by hydrogen and disulphide bonds, then fibre– like structure is formed. Such proteins are generally insoluble in water.
Examples - Keratin (present in hair, wool, silk) and Myosin (present in muscles), etc.
Globular Proteins - This structure results when the chains of polypeptides coil around to give a spherical shape. These are usually soluble in water.
Examples - Insulin and albumin


Primary And Secondary Structure Of Proteins
Primary structure of proteins: Each polypeptide in a protein has amino acids linked with each other in a specific sequence and it is this sequence of amino acids that is said to be the primary structure of that protein. Any change in this primary structure (the sequence of amino acids) creates a different protein.
Secondary structure of proteins: The secondary structure of a protein refers to the shape in which a long polypeptide chain can exist.
They are found to exist in two different types of structures viz. α-helix and β-pleated sheet structure.
α-Helix is one of the most common ways in which a polypeptide chain forms all possible hydrogen bonds by twisting into a right handed screw (helix) with the –NH group of each amino acid residue hydrogen bonded to the C-O of an adjacent turn of the helix.
In a β-pleated sheet structure, all peptide chains are stretched out to nearly maximum extension and then laid side by side which are held together by intermolecular hydrogen bonds. The structure resembles the pleated folds of drapery.


Tertiary And Quaternary Structure Of Proteins
The tertiary structure of proteins - represents the overall folding of the polypeptide chains i.e., further folding of the secondary structure. It gives rise to two major molecular shapes viz. fibrous and globular. The main forces which stabilise the 2° and 3° structures of proteins are hydrogen bonds, disulphide linkages, van der Waals and electrostatic forces of attraction.
Quaternary structure of proteins: Some of the proteins are composed of two or more polypeptide chains referred to as sub-units. The spatial arrangement of these subunits concerning each other is known as quaternary structure.
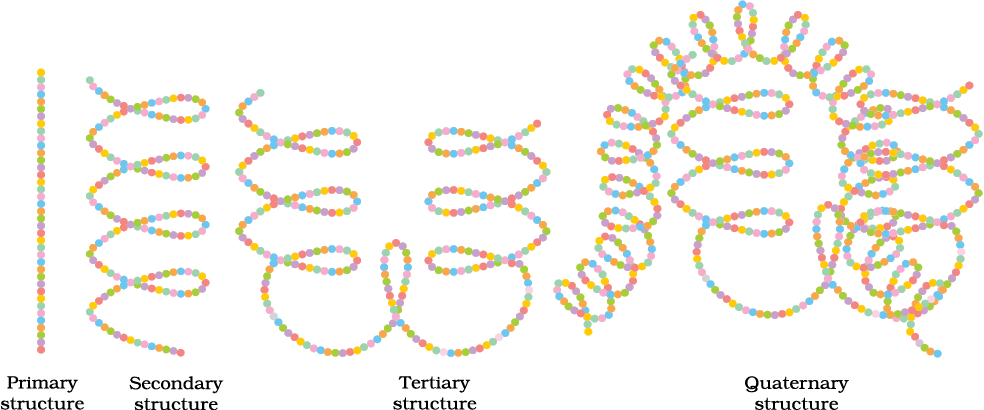


Denaturation Of Proteins
When a protein in its native form, is subjected to physical changes like temperature or pH alteration, the hydrogen bonds are disturbed. Due to this, globules unfold and helix get uncoiled and protein loses its biological activity. This is called denaturation of protein.
During denaturation secondary and tertiary structures are destroyed but primary structure remains intact.
Examples -
- The coagulation of egg white on boiling
- Curdling of milk which is caused due to the formation of lactic acid by the bacteria present in milk.


Enzymes Introduction
Enzymes are biocatalysts, which are high molecular weight proteinous compounds. It enhances the reactions which occur in the body during various life processes. Almost all the enzymes are globular proteins.
They are generally named after the compound or class of compounds upon which they work. For example, the enzyme that catalyses hydrolysis of maltose into glucose is named as maltase.
Enzymes are needed only in small quantities for the progress of a reaction. Similar to the action of chemical catalysts, enzymes are said to reduce the magnitude of activation energy.


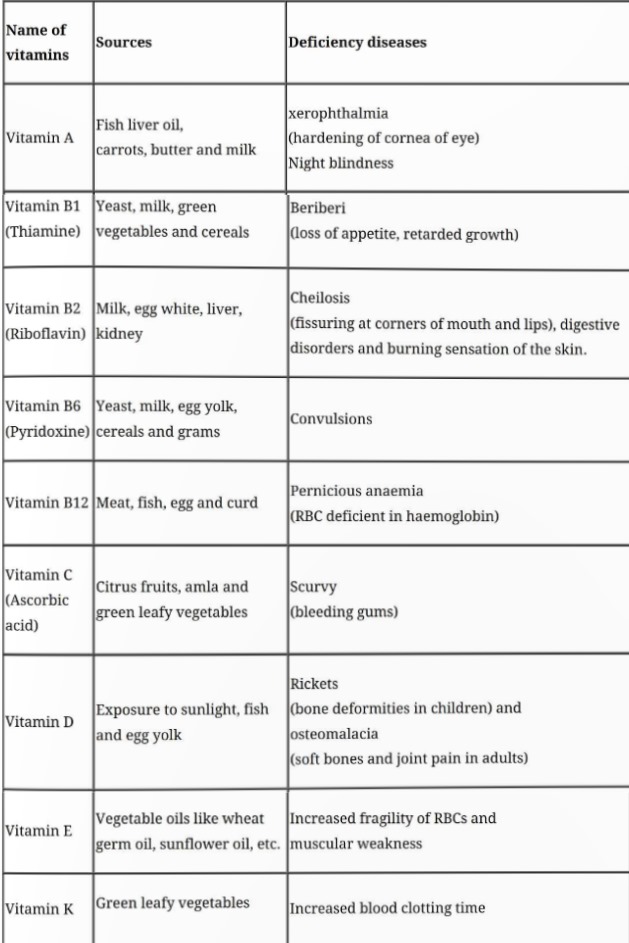
Vitamins
Fat soluble vitamins: Vitamins which are soluble in fat and oils but insoluble in water. These are vitamins A, D, E and K. They are stored in liver and adipose (fat storing) tissues.
Water soluble vitamins: B group vitamins and vitamin C are soluble in water so they are grouped together. Water soluble vitamins must be supplied regularly in diet because they are readily excreted in urine and cannot be stored (except vitamin B12) in our body.


DNA And RNA
Complete hydrolysis of DNA (or RNA) yields a pentose sugar, phosphoric acid and nitrogen containing heterocyclic compounds (called bases).
In DNA molecules, the sugar moiety is β-D-2-deoxyribose, whereas in RNA molecule, it is β-D-ribose.
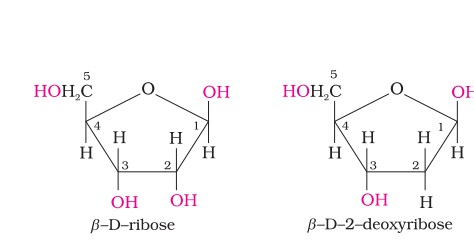
DNA contains four bases i.e. adenine (A), guanine (G), cytosine (C) and thymine (T).
RNA also contains four bases, adenine (A), guanine (G), cytosine (C) and uracil (U).


Structure - Nucleoside And Nucleotide
Deoxyribose and a nitrogenous base together form a nucleoside. A nucleoside and a phosphate together form a nucleotide.
Nucleoside = deoxyribose + nitrogenous base
Nucleotide = deoxyribose + nitrogenous base + phosphate
= nucleoside + phosphate



Structure - Continuation
Formation of a dinucleotide -
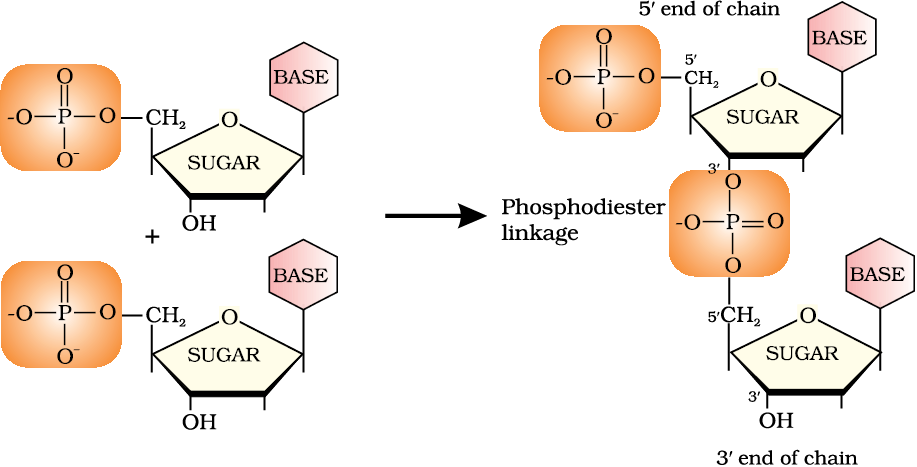
Information regarding the sequence of nucleotides in the chain of a nucleic acid is called its primary structure.
Two nucleic acid chains are wound about each other and held together by hydrogen bonds between pairs of bases. Adenine forms hydrogen bonds with thymine, whereas cytosine forms hydrogen bonds with guanine.
Nucleic acids also have a secondary structure.
RNA molecules are of three types and they perform different functions; they are named as messenger RNA (m-RNA), ribosomal RNA (r-RNA) and transfer RNA (t-RNA).


Hormones
Hormones are molecules that act as intercellular messengers. These are produced by endocrine glands in the body and are released directly in the bloodstream which transports them to the site of action.
Some are -
- Epinephrine and norepinephrine mediate responses to external stimuli.
- Growth hormones and sex hormones play a role in growth and development, like estrogen, progestrone, testosterone, etc.
- Thyroxine produced in the thyroid gland is an iodinated derivative of amino acid tyrosine.
- Steroid hormones are produced by the adrenal cortex and gonads (testes in males and ovaries in females). Hormones released by the adrenal cortex play very important role in the functions of the body.
- Glucocorticoids control carbohydrate metabolism, modulate inflammatory reactions, and are involved in reactions to stress.
- The mineralocorticoids control the level of excretion of water and salt by the kidneys.


 beeTokens
beeTokens 


